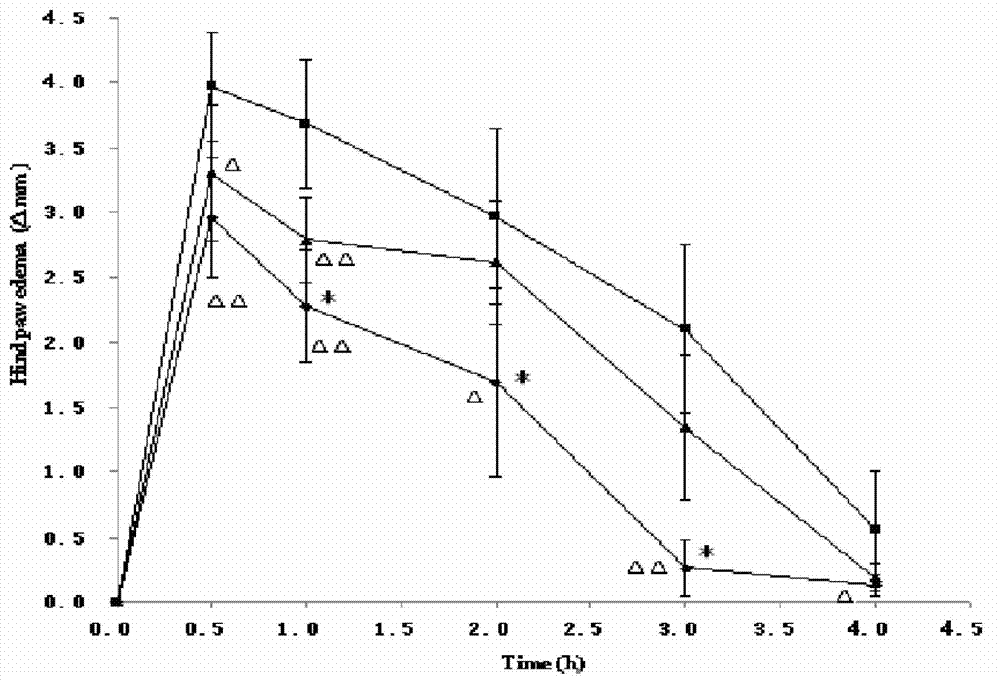
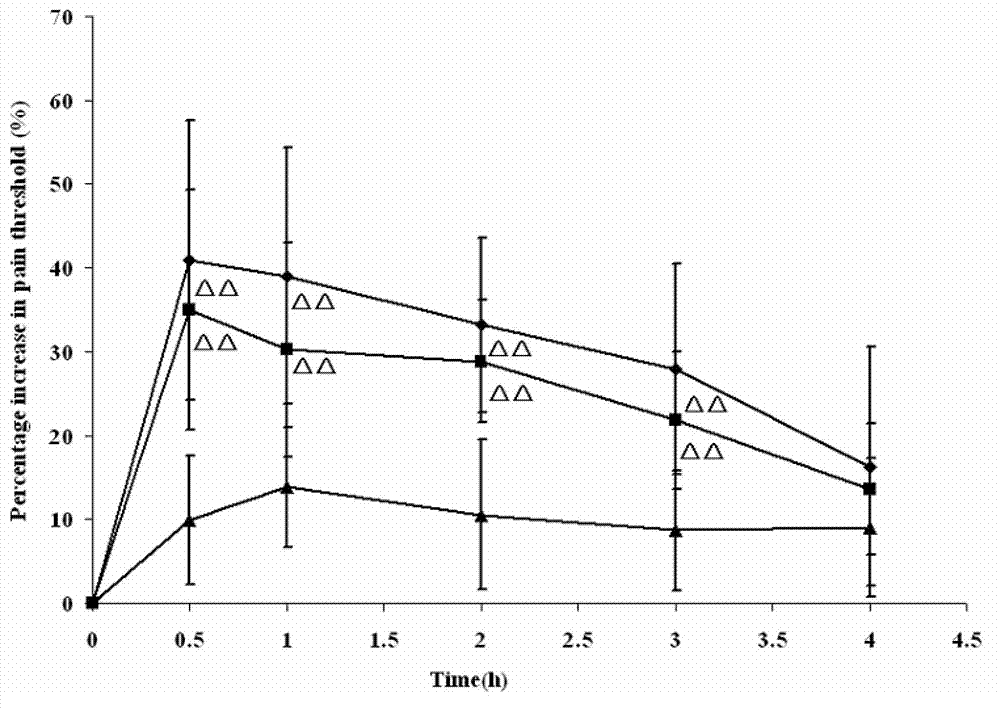
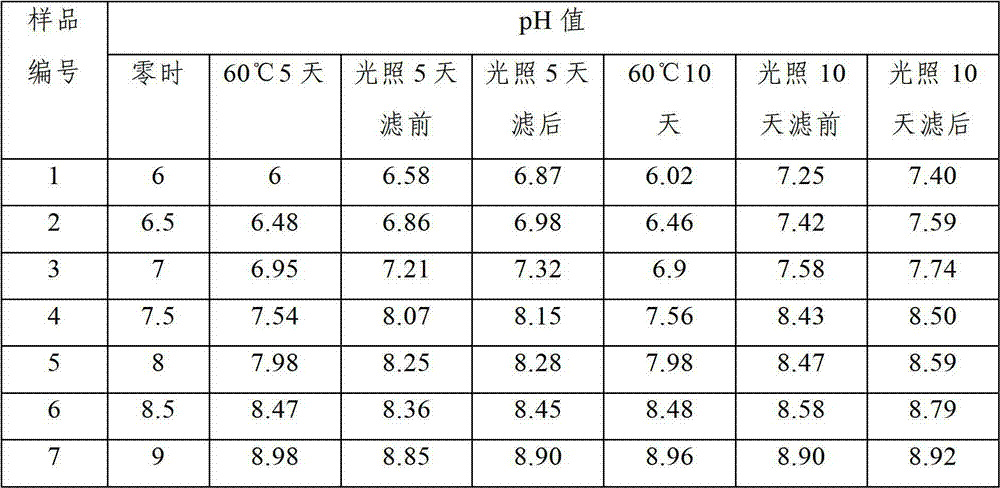
Dexketoprofen injection and preparation method thereof
A technology of dexketoprofen and injection, which is applied in the field of medicine, can solve the problems of increasing the route of drug administration, small dosage, and small side effects, and achieves the effect of reducing the process of arteriosclerosis, with small dosage and small side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The dexketoprofen injection of the present embodiment comprises dexketoprofen, L-arginine and water for injection, wherein every 1000mL of the dexketoprofen injection contains 25g dexketoprofen; The mol ratio of described dexketoprofen and L-arginine is 1: 1.
[0029] The preparation method of the dexketoprofen injection of the present embodiment is:
[0030] Step 1, adding 3 times the mass percent concentration of the bulk drug quality to the oral grade dexketoprofen bulk drug is 45% ethanol, and heating to reflux at a temperature of 50°C makes the dexketoprofen bulk drug completely dissolve , then add 0.2% activated carbon to the dissolved solution, heat it for 10 minutes and filter it while it is hot, place the filtered filtrate at -4°C overnight or cool it with cold water to precipitate crystals, and filter it with suction to obtain purified dexketorol Fern;
[0031] Step 2. Dissolve 4.29g of L-arginine in 200mL of water for injection, then add 6.25g of the purifi...
Embodiment 2
[0035] The dexketoprofen injection of the present embodiment comprises dexketoprofen, lysine and water for injection, wherein every 1000mL of the dexketoprofen injection contains 25g dexketoprofen; The mol ratio of ketoprofen and lysine is 1: 0.5.
[0036] The preparation method of the dexketoprofen injection of the present embodiment is:
[0037] Step 1, adding 5 times the mass percentage concentration of the raw material drug to the oral grade dexketoprofen raw material drug is 40% ethanol, and heating and refluxing at a temperature of 40°C makes the dexketoprofen raw material drug completely dissolve , then add 0.5% activated carbon of the solution mass to the dissolved solution, keep warm for 10 minutes and filter while it is hot, place the filtered filtrate at 4°C overnight or cool it with cold water to precipitate crystals, filter with suction to obtain purified dexketoprofen ;
[0038] Step 2. Dissolve 1.8 g of lysine in 200 mL of water for injection, then add 6.25 g ...
Embodiment 3
[0042] The dexketoprofen injection of the present embodiment comprises dexketoprofen, histidine and water for injection, wherein every 1000mL of the dexketoprofen injection contains 25g dexketoprofen; The molar ratio of ketoprofen to histidine is 1: 1.5.
[0043] The preparation method of the dexketoprofen injection of the present embodiment is:
[0044] Step 1, adding 2 times the mass percentage concentration of the raw material drug to the oral grade dexketoprofen raw material drug is 50% ethanol, and heating and refluxing at a temperature of 60 ° C makes the dexketoprofen raw material drug completely dissolved , then add activated carbon of 0.05% solution mass to the dissolved solution, keep warm for 15min and filter while it is hot, place the filtered filtrate at 4°C overnight or cool it with cold water to precipitate crystals, filter with suction to obtain purified dexketoprofen ;
[0045] Step 2. Dissolve 5.71 g of histidine in 200 mL of water for injection, then add 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com