Dexketoprofen Tromethamine Sustained Release Tablets for Gastric Retention
A technology of dexketoprofen tromethamine and sustained-release tablets, applied in the field of medicine, can solve the problems of inconvenience of taking medicine, gastrointestinal ulcer or bleeding symptoms, poor absorption and the like of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
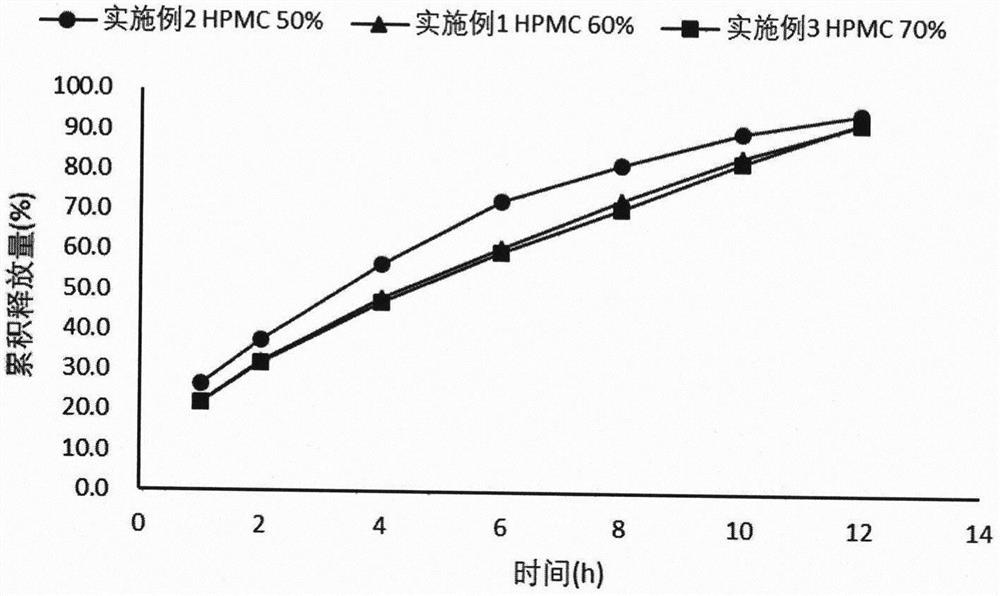
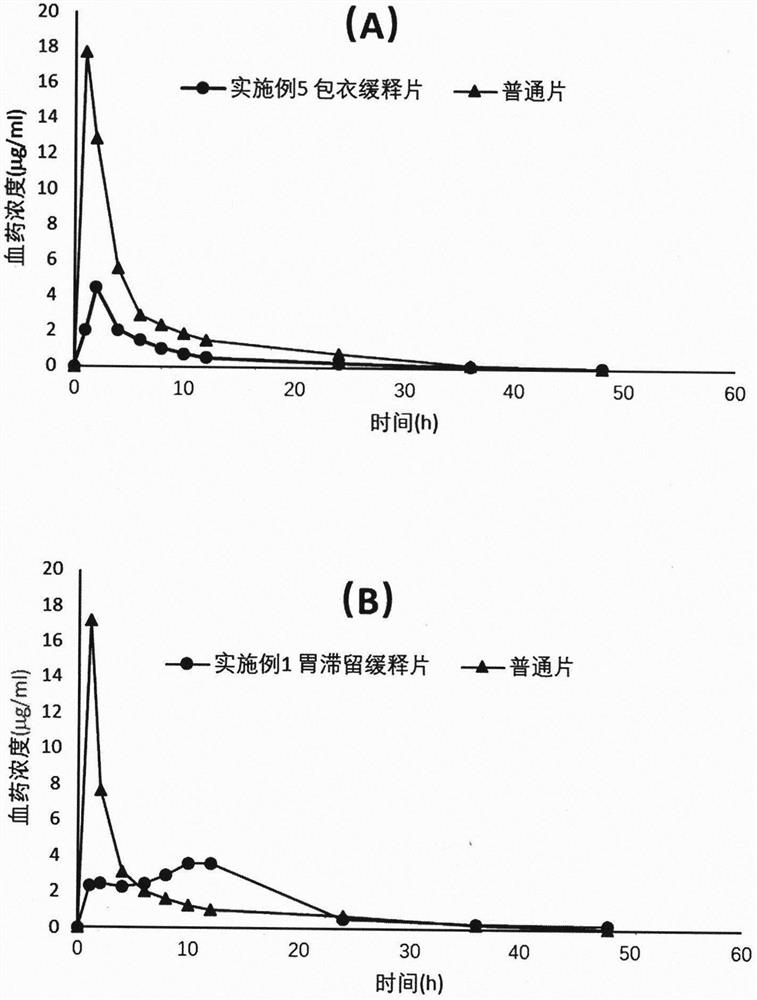
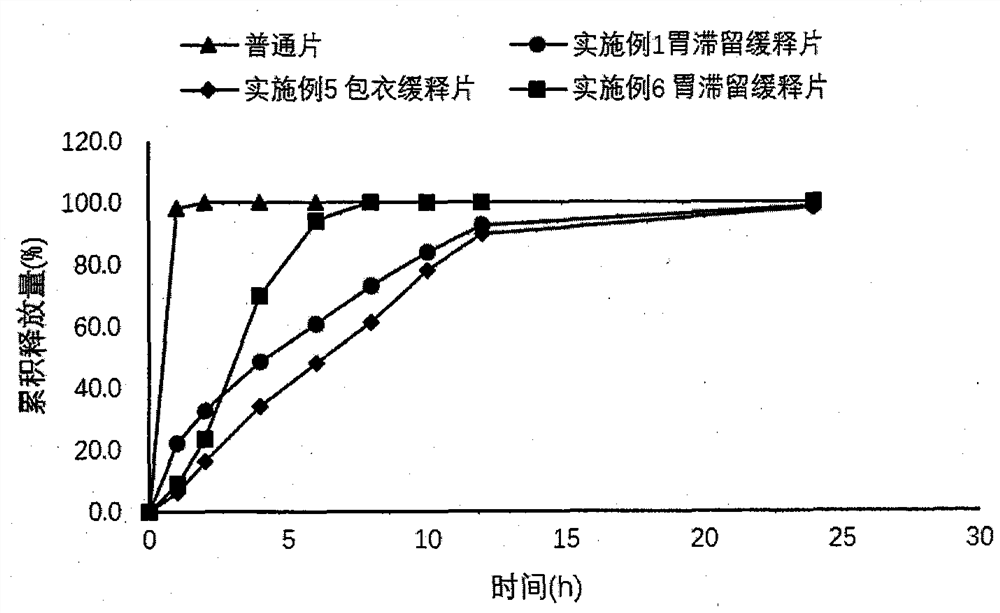
[0025] Embodiment 1 Dexketoprofen tromethamine gastric retention sustained-release tablet
[0026] Take by weighing 25g of dexketoprofen tromethamine, HPMC-K100M 166.7g, microcrystalline cellulose 83.3g, magnesium stearate 2.8g, mix homogeneously, tabletting machine tabletting, every piece contains medicine 25mg (by right Ketoprofen meter), tabletting pressure 5kg.
Embodiment 2
[0027] Embodiment 2 Dexketoprofen tromethamine gastric retention sustained-release tablet
[0028] Take by weighing 2.5g of dexketoprofen tromethamine, HPMC-K100M 13.89g, microcrystalline cellulose 11.11g, magnesium stearate 0.28g, mix homogeneously, tabletting machine tabletting, every tablet contains medicine 25mg (press Dexketoprofen meter), tabletting pressure 5kg.
Embodiment 3
[0029] Embodiment 3 Dexketoprofen Tromethamine Gastric Retention Sustained Release Tablets
[0030] Take by weighing 2.5g of dexketoprofen tromethamine, HPMC-K100M 19.44g, microcrystalline cellulose 5.56g, magnesium stearate 0.28g, mix homogeneously, tabletting machine tabletting, every tablet contains medicine 25mg (by Dexketoprofen meter), tabletting pressure 5kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com