Compound pharmaceutical composition of tapentadol hydrochloride and dexketoprofen trometamol
A technology of dexketoprofen trometamol and tapentadol hydrochloride, which is applied in the field of analgesic pharmaceutical compositions, and can solve problems such as no medicinal use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
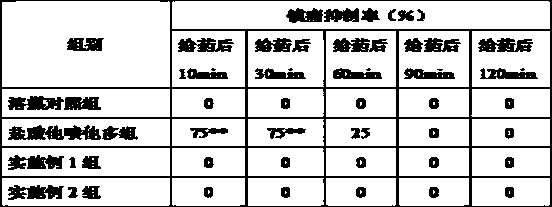
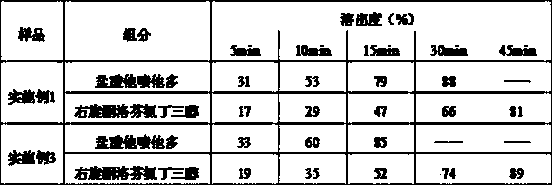
Image
Examples
Embodiment 1
[0025] Example 1 37.5mg / 12.5mg Tapentadol Hydrochloride Dexketoprofen Tromethamine Tablets
[0026] 1. Prescription
[0027] Table 1 Composition of 37.5mg / 12.5mg tapentadol hydrochloride dexketoprofen tromethamine tablets prescription (1000 tablets)
[0028]
[0029] 2. Preparation process
[0030] (1) Tapentadol hydrochloride passed through a 80-mesh sieve, lactose monohydrate, microcrystalline cellulose, and hydroxypropyl methylcellulose passed through a 60-mesh sieve, and put into a V-type mixer and mixed evenly;
[0031] (2) Put the above mixed powder into the wet mixing granulator, add purified water slowly, and shear granulate;
[0032] (3) Dry the granules at 60°C;
[0033] (4) The dried granules were sieved with 24 meshes to obtain granule 1;
[0034] (5) Dexketoprofen trometamol is passed through an 80-mesh sieve and silicon dioxide is put into a V-type mixer and mixed evenly;
[0035] (6) After passing through a 60-mesh sieve, cornstarch and sodium carboxym...
Embodiment 2
[0039] Example 2 75mg / 25mg Tapentadol Hydrochloride Dexketoprofen Tromethamine Tablets
[0040] 1. Prescription
[0041] Table 2 75mg / 25mg tapentadol hydrochloride dexketoprofen tromethamine tablets prescription composition (1000 tablets)
[0042]
[0043] 2. Preparation process
[0044](1) Tapentadol hydrochloride passed through a 80-mesh sieve, lactose monohydrate, microcrystalline cellulose, and hydroxypropyl methylcellulose passed through a 60-mesh sieve, and put into a V-type mixer and mixed evenly;
[0045] (2) Put the above mixed powder into the wet mixing granulator, add purified water slowly, and shear granulate;
[0046] (3) Dry the granules at 60°C;
[0047] (4) The dried granules were sieved with 24 meshes to obtain granule 1;
[0048] (5) Dexketoprofen trometamol is passed through an 80-mesh sieve and silicon dioxide is put into a V-type mixer and mixed evenly;
[0049] (6) After passing through a 60-mesh sieve, cornstarch and sodium carboxymethyl cellu...
Embodiment 3
[0053] Example 3 37.5mg / 12.5mg Tapentadol Hydrochloride Dexketoprofen Tromethamine Capsules
[0054] 1. Prescription
[0055] Table 3 37.5mg / 12.5mg Tapentadol Hydrochloride Dexketoprofen Tromethamine Capsules Prescription Composition (1000 capsules)
[0056]
[0057] 2. Preparation process
[0058] (1) Tapentadol hydrochloride passed through a 80-mesh sieve, lactose monohydrate, microcrystalline cellulose, and hydroxypropyl methylcellulose passed through a 60-mesh sieve, and put into a V-type mixer and mixed evenly;
[0059] (2) Put the above mixed powder into the wet mixing granulator, add purified water slowly, and shear granulate;
[0060] (3) Dry the granules at 60°C;
[0061] (4) The dried granules were sieved with 24 meshes to obtain granule 1;
[0062] (5) Pass dexketoprofen trometamol through a 80-mesh sieve, pass through a 60-mesh sieve with cornstarch and sodium carboxymethylcellulose, and mix them evenly in a V-type mixer;
[0063] (6) Put the above mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com