Method for preparing dexketoprofen
A technology of dexketoprofen and amidoketone, which is applied in the field of bioengineering, can solve problems such as low conversion rate or optical purity, and failure to meet production requirements, and achieve the effect of improving catalytic reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Construction of amidase AMD02 Escherichia coli expression strain
[0035] According to the amino acid sequence of Klebsiella amidase AMD02, the sequence is shown in SEQ ID NO:2. The gene was cloned at the NcoI and HindIII sites of pET28a, and the nucleic acid sequence was optimized according to the E. coli codons. The optimized nucleic acid sequence is shown below and was submitted to General Biosystems (Anhui) Co., Ltd. for synthesis.
[0036] ATGGCAATTCAGCGTCCGACCGCAGAACAGCTGCAGGAACTGGCAAGCCGTCTGCATATTAGCCTGACCACCGCACAGGCGGAAGAATATCTGGCTGTGATGCAGGCTAATTTTGATGCATATGATCTGATTGATAGTCTGCCGGATGATATTCCGGAAGTGCGCTATCCGCGTGCAGCAGGTTATCGTCCGACCGGCGAAGATAATCCGCTGAATGCATGGTATTATAAAACCGAAGTGAAAGGTGCAGCTACCGGTGCACTGGCTGGCCGCACCATTGCCCTGAAAGATAATGTTTCTCTGGCCGGCGTGCCGATGATGAATGGCGCCAGTACCCTGGAAGGCTTTGTTCCGAGTTGCGATGCGACCGTTGCAACCCGTCTGCTGGATGCCGGCGCCACCATTCTGGGTAAAGCGACCTGCGAACATTTTTGCCTGAGCGGTGGTTCTCATACCTCAGATCCGGCCCCGGTTCATAATCCGCATCGCCACGGTTATTCTAGCGGCGGCAGTTCATCTGGTAGTGCGGCA...
Embodiment 2
[0039] Example 2 Shake flask induced expression of amidase AMD02
[0040] Pick a single colony on the transformation plate to 5mL LB+Kan liquid medium (10g / L peptone, 5g / L yeast powder, 10g / L sodium chloride, 50μg / mL kanamycin), 37℃250rpm / min Cultivate overnight under the conditions, about 12h.
[0041] Take the activated bacteria solution overnight, transfer 500μl to fresh 50mL LB+Kan liquid medium, and incubate at 37℃250rpm / min until the OD reaches 0.5-0.8.
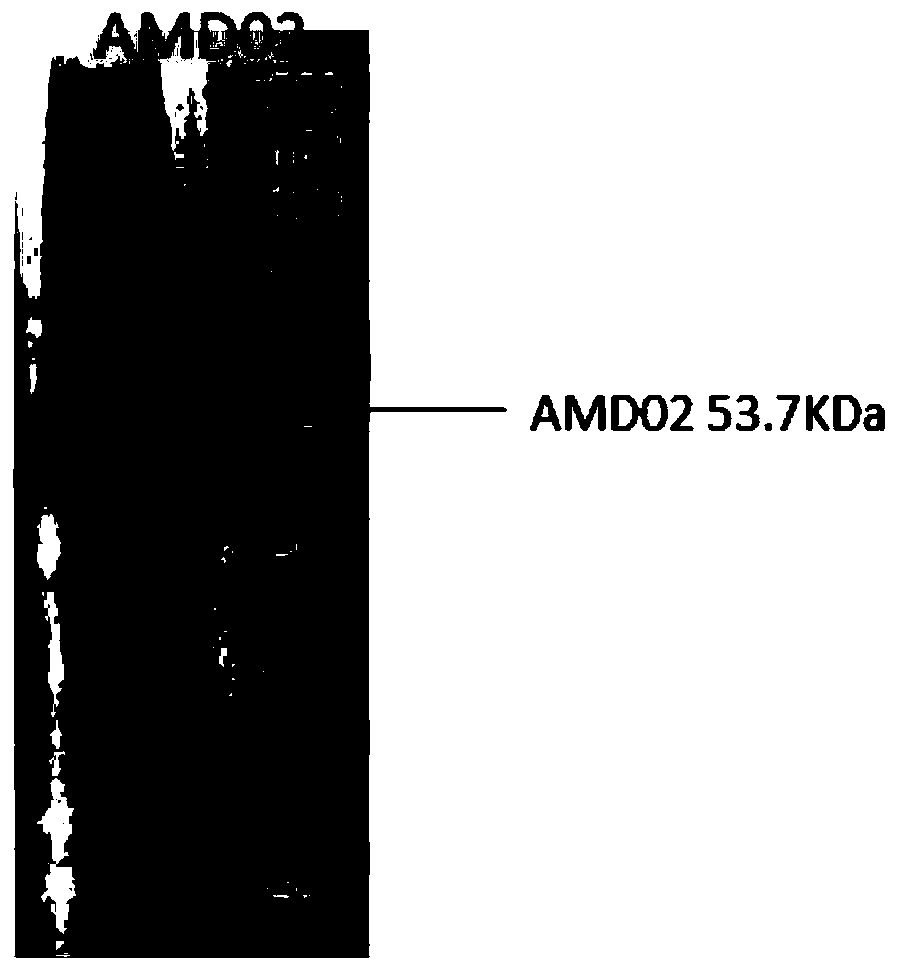
[0042] Take the cultured AMD02 bacterial solution, add IPTG with a final concentration of 1 mM, and culture for 5 hours at 30°C and 250 rpm / min.
[0043] After the induction, all the bacterial liquid was collected and centrifuged at 8000 prm / min and 4 degrees Celsius to collect the bacterial cells. Take appropriate bacteria to break the wall to detect protein expression.
[0044] See for protein induction figure 1 .
Embodiment 3
[0045] Example 3 50L tank fermentation of amidase AMD02
[0046] Take the overnight activated bacterial solution in Example 2, transfer 1 mL to a fresh 1000 mL LB+Kan liquid medium, incubate at 37°C for about 10 hours at 250 rpm / min, and then inoculate a 50L fermentor (fermentation medium: 121g glucose monohydrate , Yeast extract powder 500g, yeast peptone 250g, NaCl100g, Na 2 HPO 4 25g, MgSO 4 .7H 2 O 20g, KH 2 PO 4 100g, (NH 4 ) 2 SO 4 50g, 55g of citric acid monohydrate, 75g of NaOH, 25mL of GPE defoamer. Sterilize at 118°C for 30 minutes, and the volume after elimination is 25L).
[0047] Basic culture stage: control the culture temperature to 37.0℃, the aeration volume is 1:1vvm, the dissolved oxygen level of the fermentation broth is adjusted to be higher than 50% by stirring and aeration, and the tank pressure is 40-70Kpa.
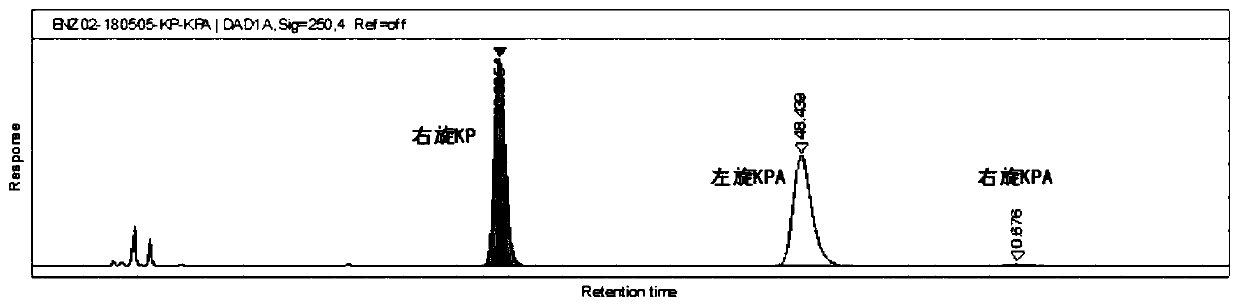
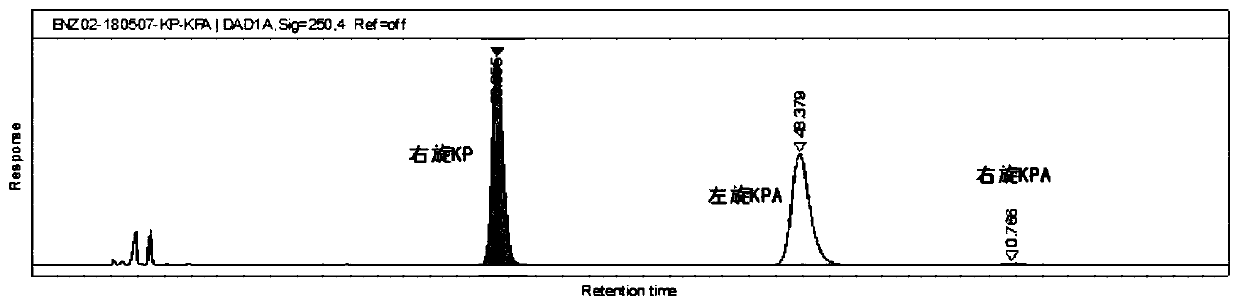
[0048] Glucose supplement stage: start to add glucose solution when the pH of the fermentation broth rebounds to 7.15. The initial sugar supplement rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com