Amidase variant with improved specific activity and application thereof
An amidase and amidase-describing technology, applied in the field of molecular biology, can solve the problems of large amount of enzyme added and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
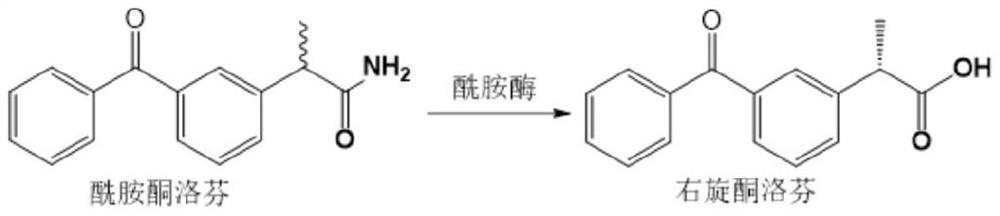
[0084]The present invention provides a method of preparing an amide enzyme variant, and an amide enzyme variant is expressed in E. coli, an enzyme-catalytic reaction is performed using E. coli, wherein the pathogenic pathway is used to catalyze the ketone Loven synthesis:
[0085]
Embodiment 1
[0087] Example 1 Construction of Expression strains of amidase AMD03 E. coli
[0088] According to Rhodococcus Erythropolis MP50 amide AMD03 amino acid sequence, the sequence is shown in SEQ ID NO: 1. Optimization is optimized in accordance with E. coli codons, and sequences are shown in SEQ ID NO: 2. An RBS site is added in front of the amide enzyme AMD03 nucleotide sequence, and the selected RBS sequence is submitted as aaagaggagaaa, and the SUMCE is submitted for synthesis.
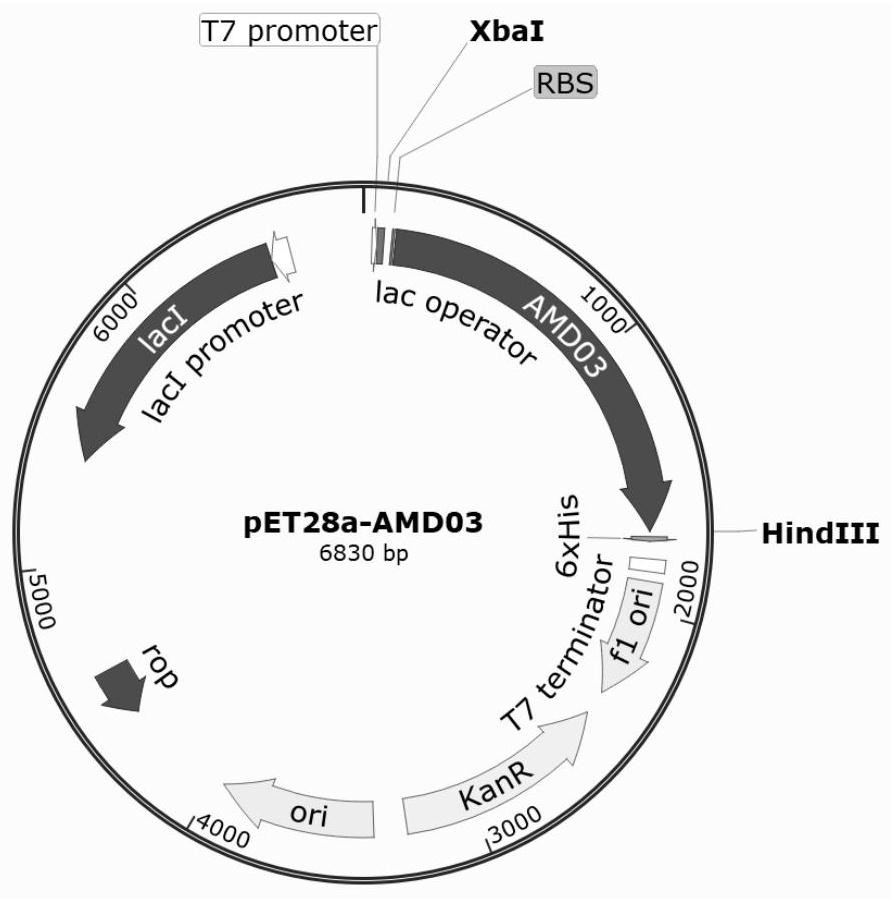
[0089] Then, by gene recombination technique, the above-described synthetic nucleotide sequence was cloned to the XBAI of the PET28A vector, and PET28A-AMD03 plasmid, plasmid spectrum figure 1 Indicated. PET28A-AMD03 plasmid transforms BL21 (DE3) induced state cells, conversion of LB + KAN plate (10 g / L protein, 5g / L yeast powder, 10 g / l sodium chloride, 50 μg / ml kazza) Mythromycin, 15g / L agar powder), 37 ° C overnight culture. The resulting transformation was named BL21-AMD03 strain after the PC...
Embodiment 2
[0090] Example 2 induced expression of amidase AMD03
[0091] The BL21-AMD03 strain freezed -80 ° C-80 ° C was separated from the LB + KAN flat scribe line. Picking the single bacteria on the conversion plate to 5 mL liquid LB + KAN (10 g / L protein, 5g / L yeast powder, 10 g / l sodium chloride, 50 μg / ml kanamycin) medium, 37 ° C, 250g / Overnight culture under min, about 12 h.
[0092] Take overnightly activated bacterial liquid, 2ml transfer to fresh 200ml liquid LB + KAN, cultured under 37 ° C, 250 g / min until OD 600 The value reached 0.5-0.8.
[0093] The cultured bacterial solution was taken, and the final concentration of 1 mM IPTG, 30 ° C, 250 g / min, and cultured under the conditions of 5 h.
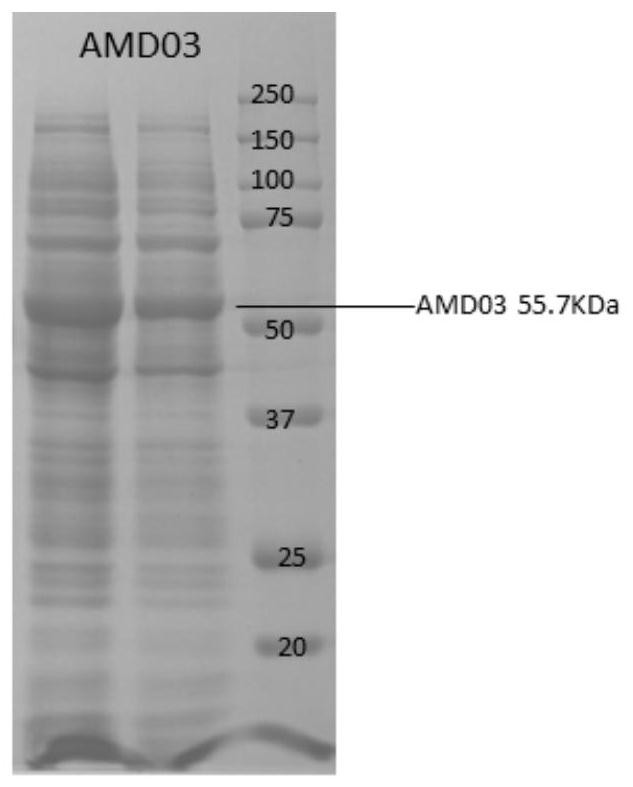
[0094] After the end of the induction, the total bacterial liquid was collected at 4000 g / min, 4 ° C. Take appropriate bacteria to perform ultrasonic breakage treatment, centrifugation of 10 min at 4 ° C, and take the supernatant to detect protein expression by SDS-PAGE, suc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com