A kind of dexketoprofen tromethamine sustained-release tablet and preparation method thereof
A technology for dexketoprofen trometamol and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of decreased bioavailability and failure to achieve Treatment effect, damage to the structure and balance of hypromellose, etc., to achieve the effect of simple process and suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
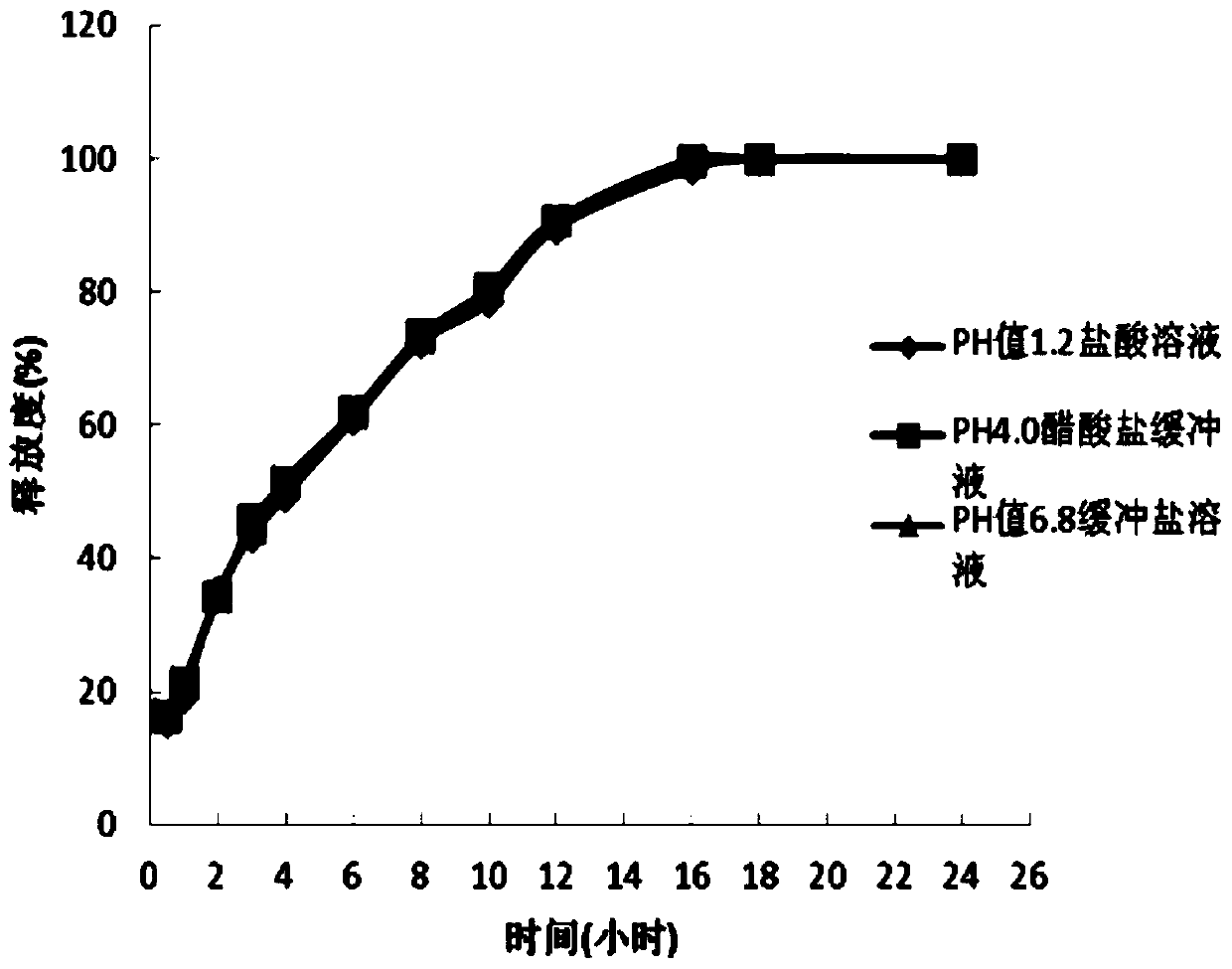
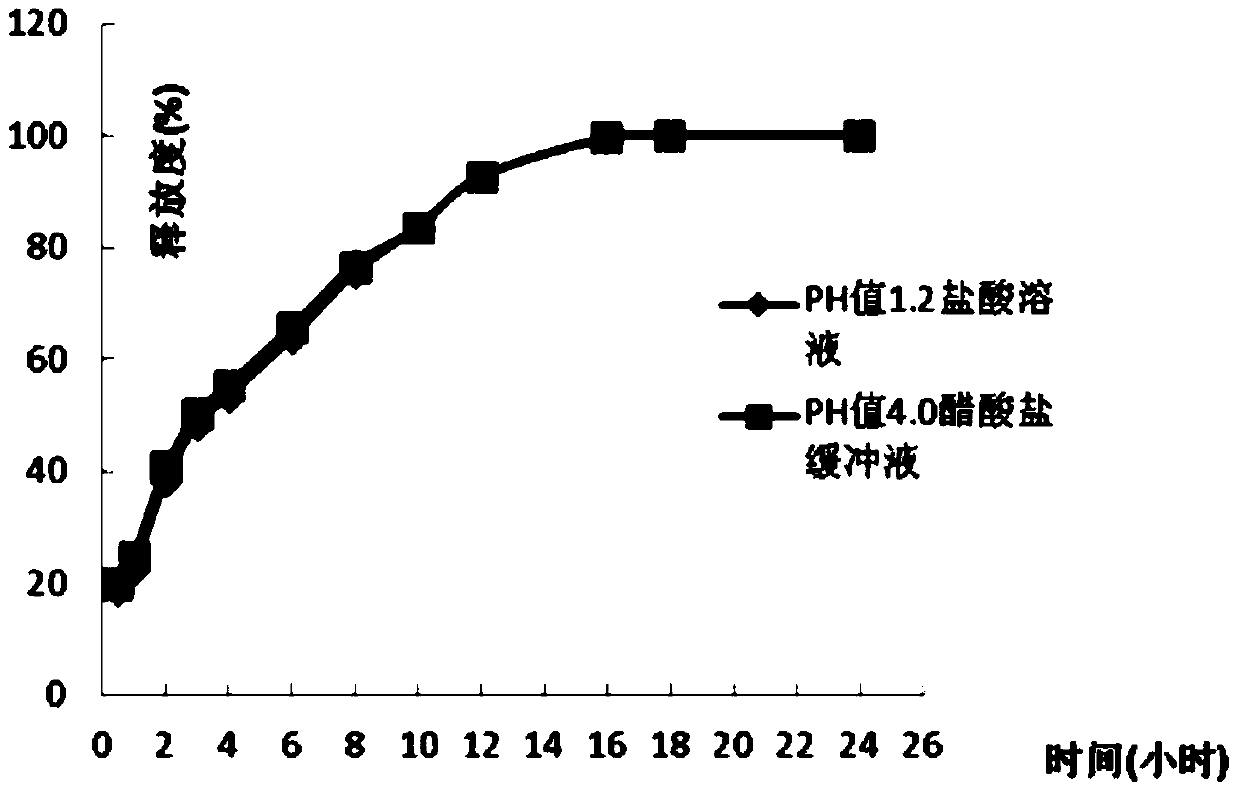
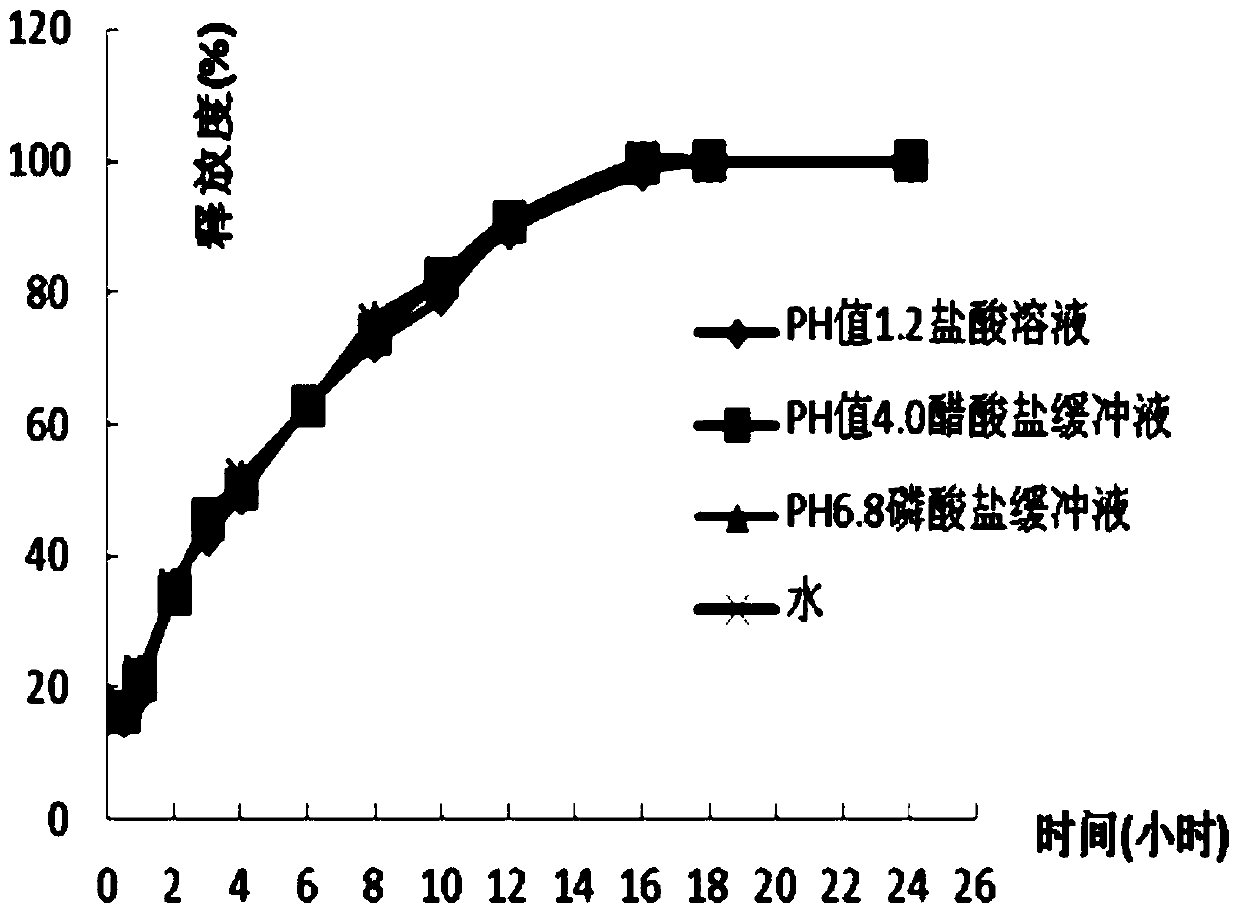
[0036] According to the amount of 1000 tablets, weigh 73.8g of dexketoprofen trometamol, 82.5g of hypromellose K15M, 2.5g of silicon dioxide, and 3.5g of magnesium stearate and mix evenly, and partly pregelatinized starch PC Mix -10 and Eudragit EPO in a ratio of 1:2.1 (w / w), mix for 20-30 minutes until the powder is fully adhered together, set aside, and then weigh 150g of the composition, and then mix all the materials evenly, according to each Tablet 312.3 mg is compressed, and the compression hardness is controlled at 6-12Kg.
Embodiment 2
[0038] According to the amount of 1000 tablets, weigh 73.8g of dexketoprofen trometamol, 92.5g of hypromellose K15M, 3.3g of silicon dioxide, and 3.3g of magnesium stearate and mix evenly, and partly pregelatinized starch PC -10 and Eudragit EPO are mixed in a ratio of 1:2.6 (w / w), and mixed for 20 to 30 minutes until the powder is fully adhered together, set aside, then weigh 140.8g of the composition, and mix all the materials evenly, according to each Tablet 313.7 mg is compressed, and the compression hardness is controlled at 6-12Kg.
Embodiment 3
[0040] According to the amount of 1000 pieces, take by weighing 73.8g of dexketoprofen tromethamine, hypromellose K15M 82.5g, microcrystalline cellulose 20g, silicon dioxide 3.3g, magnesium stearate 3.3g and mix evenly, Partially pregelatinized starch PC-10 and Eudragit EPO were mixed in a ratio of 1:3.5 (w / w), and mixed for 20 to 30 minutes until the powders were fully adhered together, and set aside, then weighed 150g of the composition, and mixed all the materials Mix evenly, carry out tabletting according to 332.9mg per tablet, hardness is controlled at 6~12Kg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com