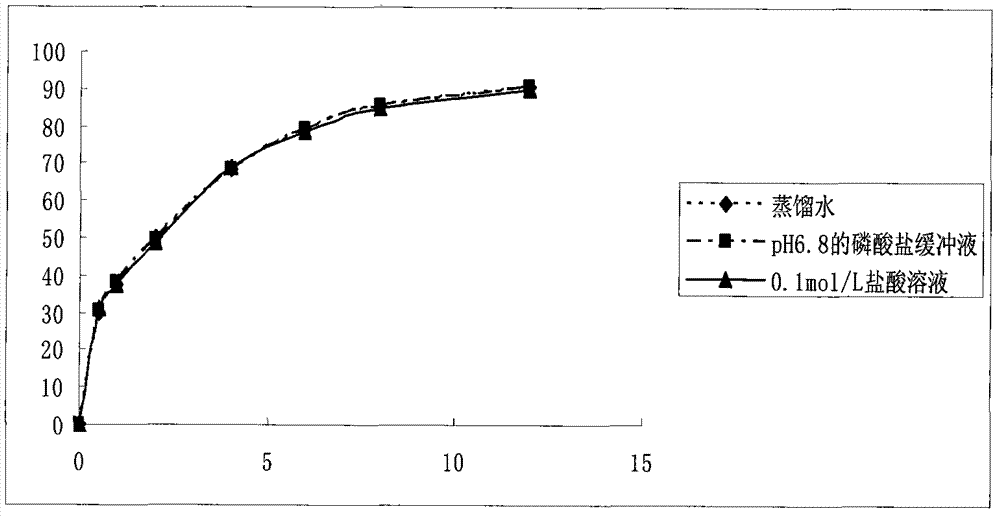
Preparation method of dexketoprofen trometamol double-layer sustained-release tablets
A technology of dexketoprofen tromethamine and sustained-release tablets, which is applied in the field of medicine and can solve the problems of large fluctuation of blood drug concentration, low blood drug concentration, slow drug release of sustained-release preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
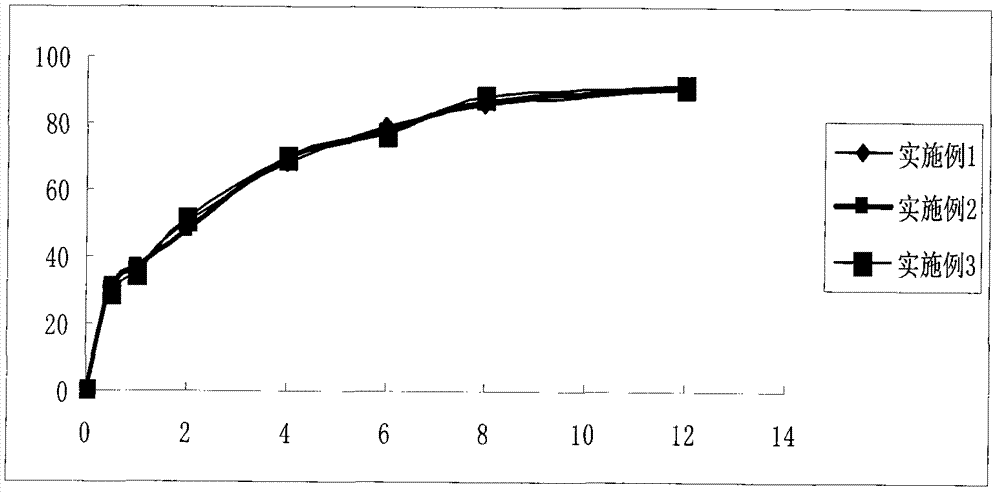
Embodiment 1
[0030] Prescription (based on 1000 tablets)
[0031] Immediate release layer:
[0032]
[0033] Sustained release layer:
[0034]
[0035]
[0036] Preparation Process
[0037] 1.1 Preparation of immediate release layer particles
[0038] Each component in the prescription was passed through a 100 mesh sieve, and the prescription amount of lactose, microcrystalline cellulose, and sodium carboxymethyl starch were thoroughly mixed, and then thoroughly mixed with dexketoprofen tromethamine, and the appropriate amount The 10% PVP K30 solution is a soft material made of a binder, granulated by a 20-mesh sieve, dried by blowing at 60°C, granulated by a 20-mesh sieve, and then mixed with magnesium stearate;
[0039] 1.2 Preparation of sustained-release layer particles
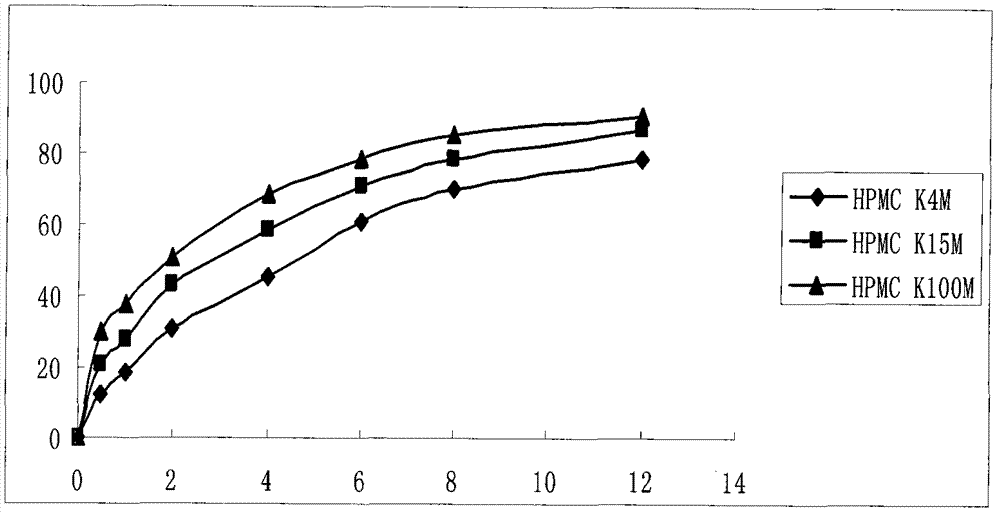
[0040] Each component in the prescription was passed through a 100 mesh sieve, and then the prescription amount of HPMC K100M, ethyl cellulose, lactose, and microcrystalline cellulose were thoroughly mixed, and then fully mixed ...
Embodiment 2
[0044] Prescription (based on 1000 tablets)
[0045] Immediate release layer:
[0046]
[0047]
[0048] Sustained release layer:
[0049]
[0050] Preparation Process
[0051] 1.1 Preparation of immediate release layer particles
[0052] Each component in the prescription was passed through a 100 mesh sieve, and the prescription amount of lactose, microcrystalline cellulose, and sodium carboxymethyl starch were thoroughly mixed, and then thoroughly mixed with dexketoprofen tromethamine, and the appropriate amount The 10% PVP K30 solution is a soft material made of a binder, granulated by a 20-mesh sieve, dried by blowing at 60°C, granulated by a 20-mesh sieve, and then mixed with magnesium stearate;
[0053] 1.2 Preparation of sustained-release layer particles
[0054] Each component in the prescription was passed through a 100 mesh sieve, and then the prescription amount of HPMC K100M, ethyl cellulose, lactose, and microcrystalline cellulose were thoroughly mixed, and then fully mixed ...
Embodiment 3
[0058] Prescription (based on 1000 tablets)
[0059] Immediate release layer:
[0060]
[0061] Sustained release layer:
[0062]
[0063] Preparation Process
[0064] 1.1 Preparation of immediate release layer particles
[0065] Each component in the prescription was passed through a 100 mesh sieve, and the prescription amount of lactose, microcrystalline cellulose, and sodium carboxymethyl starch were thoroughly mixed, and then thoroughly mixed with dexketoprofen tromethamine, and the appropriate amount The 10% PVP K30 solution is a soft material made of a binder, granulated by a 20-mesh sieve, dried by blowing at 60°C, granulated by a 20-mesh sieve, and then mixed with magnesium stearate;
[0066] 1.2 Preparation of sustained-release layer particles
[0067] Each component in the prescription was passed through a 100 mesh sieve, and then the prescription amount of HPMC K100M, ethyl cellulose, lactose, and microcrystalline cellulose were thoroughly mixed, and then fully mixed with dexk...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com