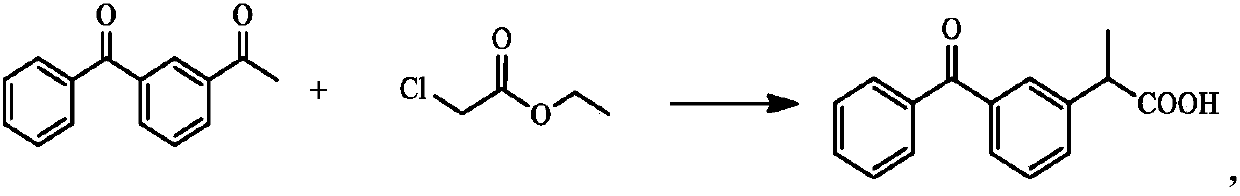
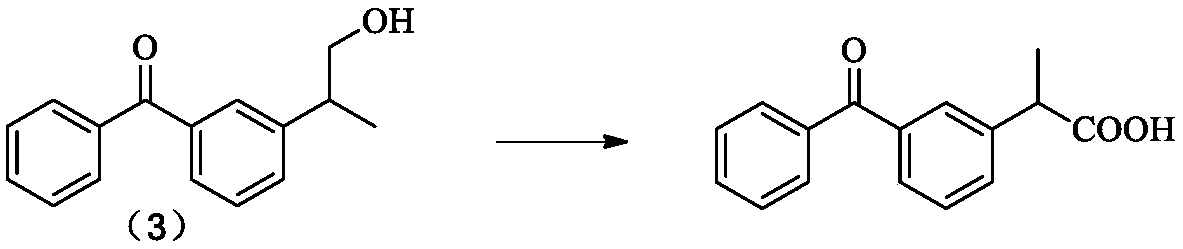
Method for synthesizing dexketoprofen intermediate
A kind of technology of dexketoprofen and synthesis method, applied in the field of medicine, can solve the problems of cumbersome operation, unfavorable industrialized large-scale production, large loss of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
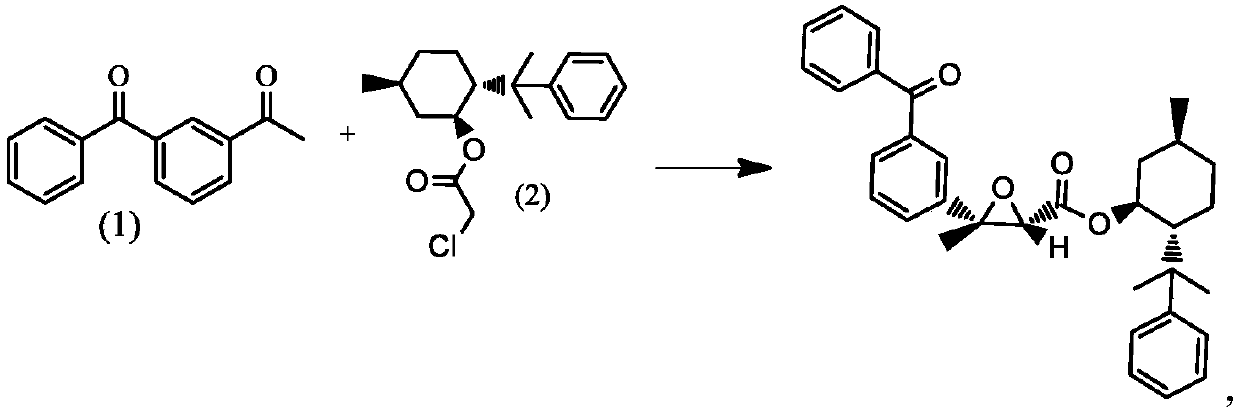
[0022]
[0023] Dissolve 8.67 g (0.0389 mol) of compound (1) and 15.00 g (0.0486 mol) of compound (2) in 300 ml of dichloromethane under nitrogen protection. The temperature was lowered to -30°C with stirring, 6.00 g of potassium tert-butoxide was added therein, and the temperature was controlled at -10 to -5°C for 3 hours. After the reaction was complete, water was slowly added dropwise at 0°C, stirred for 1 h, then left to stand, and the organic phase was obtained by liquid separation and washed with saturated brine. Add anhydrous sodium sulfate 30.00 to the organic phase to dry, 1h, and filter with suction. The filtrate was concentrated to dryness at 30°C to obtain the crude product. The crude product was mixed with ethyl acetate and n-hexane solution, beaten for 1 hour at 30°C, and 20.50 g of the refined product was obtained by suction filtration.
Embodiment 2
[0025]
[0026] Dissolve 9.76 g (0.044 mol) of compound (1) and 15.00 g (0.0486 mol) of compound (2) in 300 ml of dichloromethane under nitrogen protection. The temperature was lowered to -10°C while stirring, 6.00 g of potassium tert-butoxide was added therein, and the temperature was controlled at -10 to -5°C to react for 3 hours. After the reaction was complete, water was slowly added dropwise at 0°C, stirred for 1 h, then left to stand, and the organic phase was obtained by liquid separation and washed with saturated brine. Add anhydrous sodium sulfate 30.00 to the organic phase, dry it for 2 hours, and filter with suction. The filtrate was concentrated to dryness at 30°C to obtain a crude product, which was mixed with ethyl acetate and n-hexane solution, beaten for 2 hours at 40°C, and filtered with suction to obtain 22.43 g of a refined product.
Embodiment 3
[0028]
[0029] 11.93 g (0.0534 mol) of compound (1) and 15.00 g (0.0486 mol) of compound (2) were dissolved in 300 ml of dichloromethane under nitrogen protection. The temperature was lowered to -20°C with stirring, 6.00 g of potassium tert-butoxide was added therein, and the temperature was controlled at -10 to -5°C for 3 hours. After the reaction was complete, water was slowly added dropwise at 0°C, stirred for 1 h, then left to stand, and the organic phase was obtained by liquid separation and washed with saturated brine. Add anhydrous sodium sulfate 30.00 to the organic phase to dry, 3h, and filter with suction. The filtrate was concentrated to dryness at 30°C to obtain a crude product, which was mixed with ethyl acetate and n-hexane solution, beaten for 3 hours at 50°C, and filtered with suction to obtain 21.47 g of a refined product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com