Synthesis method of 2-hydroxy-3-methoxy-3, 3-diphenyl propionic acid
A technology of diphenylpropionic acid and its synthetic method, which is applied in the preparation of oxygen-containing compounds, chemical instruments and methods, and preparation of carboxylic acid esters. It can solve the problems of increasing hidden dangers in the quality control of intermediates and final products, and avoid genotoxicity Impurities, suitable for industrial production, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] step a
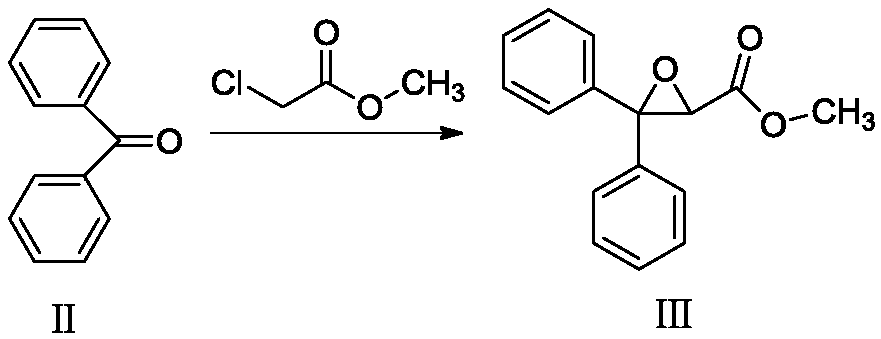
[0031] Add 20g of compound II and 23.8g of methyl chloroacetate into N,N-dimethylformamide, stir to dissolve, add 69.9g of potassium phosphate, heat to 70°C for reaction, wait until TLC detects that the reaction of compound II is complete, and cool down to room temperature , adding water to the reaction solution, adding dichloromethane for extraction, washing with saturated brine, drying over anhydrous sodium sulfate, filtering, and concentrating under reduced pressure to obtain compound III as a light yellow oil.
[0032] step b
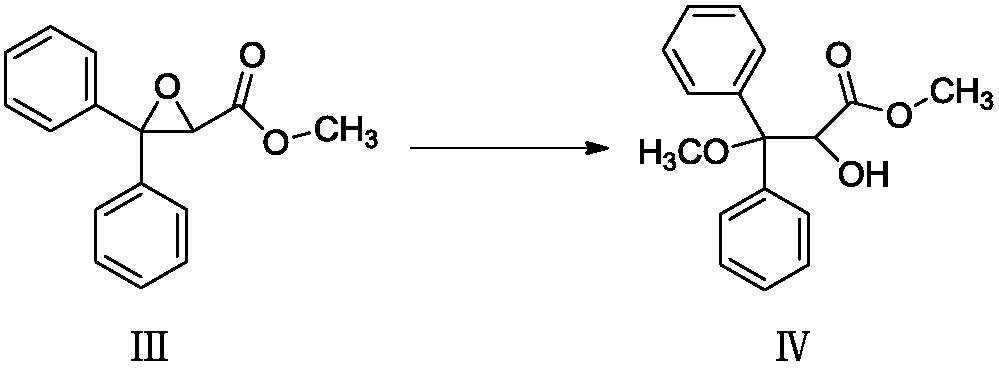
[0033] Add compound III to methanol, stir to dissolve, add 1.3g trifluoroacetic acid, control the temperature below 45°C, wait for TLC to detect that compound III has reacted completely, cool down to 0-10°C to crystallize, filter to obtain white solid compound IV, and HPLC detection showed that the purity was 99.54%, benzophenone was not detected, and the maximum single impurity was 0.10%. step c
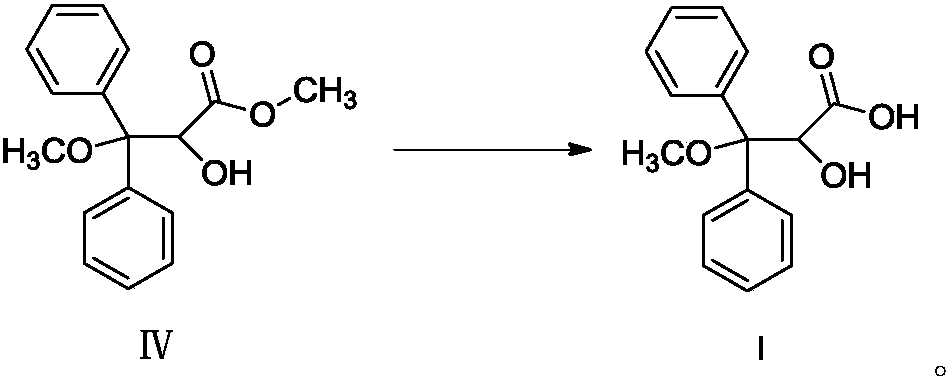
[0034] Add compound IV to 120mL ...
Embodiment 2
[0036] step a
[0037] Add 20g of compound II and 23.8g of methyl chloroacetate into N,N-dimethylformamide, stir to dissolve, add 81.5g of potassium phosphate, heat to 70°C for reaction, wait until TLC detects that the reaction of compound II is complete, and cool down to room temperature , adding water to the reaction solution, adding dichloromethane for extraction, washing with saturated brine, drying over anhydrous sodium sulfate, filtering, and concentrating under reduced pressure to obtain compound III as a light yellow oil.
[0038] step b
[0039] Add compound III to methanol, stir to dissolve, add 1.8g trifluoroacetic acid, control the temperature below 45°C, wait for TLC to detect that compound III has reacted completely, cool down to 0-10°C to crystallize, filter to obtain white solid compound IV, and HPLC detection showed that the purity was 99.46%, benzophenone was not detected, and the maximum single impurity was 0.12%. step c
[0040] Add compound IV to 120mL ...
Embodiment 3
[0042] step a
[0043]Add 20g of compound II and 26.0g of methyl chloroacetate into N,N-dimethylformamide, stir to dissolve, add 69.9g of potassium phosphate, heat to 80°C for reaction, wait until TLC detects that the reaction of compound II is complete, and cool down to room temperature , adding water to the reaction solution, adding dichloromethane for extraction, washing with saturated brine, drying over anhydrous sodium sulfate, filtering, and concentrating under reduced pressure to obtain compound III as a light yellow oil.
[0044] step b
[0045] Add compound III to methanol, stir to dissolve, add 1.8g trifluoroacetic acid, control the temperature below 40°C, wait for TLC to detect that compound III has reacted completely, cool down to 0-10°C to crystallize, filter to obtain white solid compound IV, and HPLC detection showed that the purity was 99.52%, benzophenone was not detected, and the maximum single impurity was 0.14%. step c
[0046] Add compound IV into 120mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com