Preparation process of ketoprofen
A preparation process and ketoprofen technology, applied in the field of medicine, can solve the problems of expensive raw materials, long reaction route, low total yield and the like, and achieve the effects of avoiding Grignard reaction, low equipment requirements and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
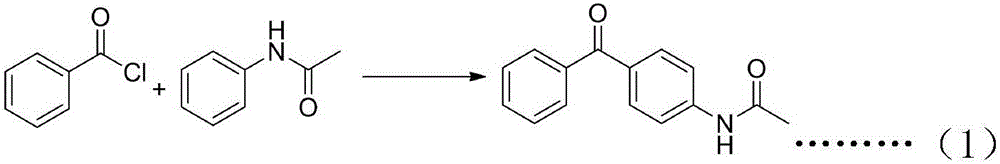
[0063] Example 1. Preparation of 4-acetamidobenzophenone (1).
[0064] 140 g of benzoyl chloride and 148 g of acetanilide were dissolved in 400 g of chloroform, 145 g of aluminum trichloride was added, and reacted at room temperature for 8 hours. After the reaction, the reaction solution was slowly poured into 500 ml of dilute hydrochloric acid ice water, and vigorously stirred while pouring. The liquid was separated, the organic layer was dried over anhydrous sodium sulfate, the solvent was recovered under reduced pressure, and petroleum ether: ethyl acetate = 1:1 was recrystallized to obtain 222 g of intermediate 4-acetamidobenzophenone (1), with a yield of 93%.
Embodiment 2
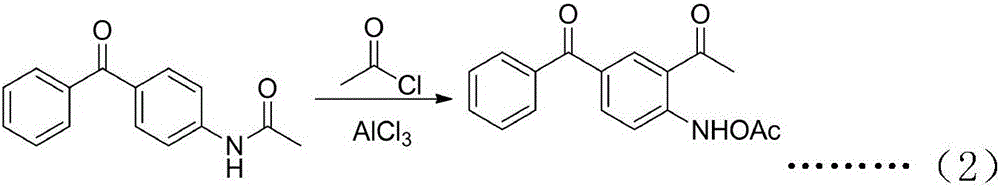
[0065] Example 2. Preparation of 2-acetylamino-5-benzoyl-acetophenone (2)
[0066] Dissolve 120g of 4-acetamidobenzophenone, 67g of aluminum trichloride in 400g of ethyl acetate, slowly add 40g of acetyl chloride dropwise under ice bath, and finish dropping in 30 minutes. The reaction solution was slowly poured into 150 ml of dilute hydrochloric acid in ice water, stirred while pouring, and separated, the organic layer was dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure. Petroleum ether: ethyl acetate = 5:3 recrystallized to obtain 2- Acetylamino-5-benzoyl-acetophenone (2) 139 g, yield 94%.
Embodiment 3
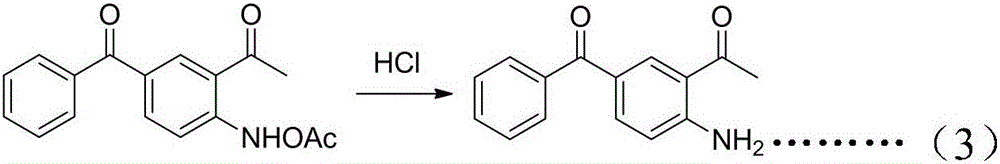
[0067] Example 3. Preparation of 2-acetyl-4-benzoylaniline (3)
[0068] 139g of 2-acetamido-5-benzoyl-acetophenone, dissolved in 200ml of ethanol, added 100ml of 40% dilute hydrochloric acid, heated to reflux for 2h, after the reaction was completed, cooled to room temperature, and added dropwise sodium carbonate solution to alkaline. Extracted with ethyl acetate, dried over anhydrous sodium sulfate, recovered the solvent under reduced pressure, and recrystallized petroleum ether: ethyl acetate = 1:1 to obtain 109.6 g of 2-acetyl-4-benzoylaniline (3), with a yield of 98% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com