Benzimidazole amide bactericide
A technology of benzimidazolone amide and agricultural fungicide, which is applied in the field of pesticides, can solve the problems of unreported antibacterial activity, and achieve the effect of less synthesis steps, high reaction yield and simple raw materials
Inactive Publication Date: 2010-06-30
PESTICIDE INST XIBEI AGRI & FORESTRY TECHUNIV
View PDF0 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This type of compound is rarely reported in the literature, and its antibacterial activity has not been reported
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
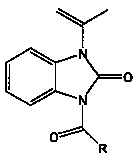
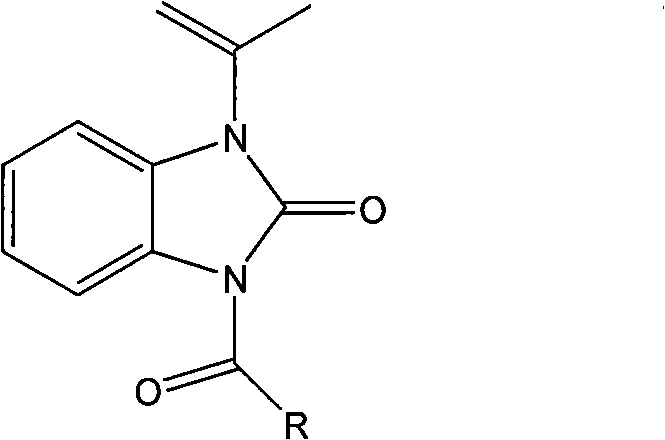
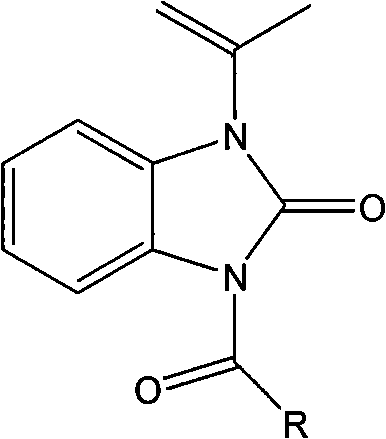
The invention relates to a novel benzimidazole amide compound and an application thereof as an agricultural bactericide, belonging to the technical field of pesticides. The compound has the structural formula disclosed in the specification, wherein R represents an alkyl group with a carbon chain length of C1-C4, specifically a methyl, an ethyl, a propyl, a normal-butyl and an isobutyl; an alkenyl with a carbon chain length of C2-C4, specifically a vinyl, an allyl, an isopropenyl and an isobutenyl; a halogenated alkyl with a carbon chain length of C1-C2, specifically a chloromethyl group, a brooethyl, a 2-chloroethane and a 2-bromomethyl; and different substituted aryl groups specifically refer to a parachloro-penyl methyl, a p-bromophenyl methyl, a p-methoxybenzene methyl, a p-nitro phenyl; and a furfuryl.
Description
technical field The technical scheme of the invention relates to a class of benzimidazolone amide fungicides, belonging to the technical field of pesticides. Background technique Crop disease is an important factor leading to the reduction of yield and quality of agricultural products, and chemical control is the main technical means of crop disease control. Organic synthetic fungicides are the main part of the current agricultural fungicides and an important part of the modern pesticide industry. However, the problem that cannot be ignored is that with the extensive use of organic synthetic fungicides, crop pathogens have shown different degrees of drug resistance or drug resistance. In order to solve this problem, it is a continuous problem faced by the pesticide industry to continuously develop various new fungicides. Since benomyl was discovered in 1968 to have excellent properties of preventing and controlling fungal diseases, the bactericidal properties of benzimida...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D235/26A01N43/52A01P3/00
Inventor 姬志勤吴文君张继文李圣坤
Owner PESTICIDE INST XIBEI AGRI & FORESTRY TECHUNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com