Modified polymer complex, complex monomer, polymer complex, and redox catalyst
A complex and polymer technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, copper organic compound, etc., can solve the problem of poor storage stability, poor manufacturing reproducibility, expensive catalyst, etc. problem, to achieve the effect of high reactivity and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0250] (Production Example 1) [Synthesis of Ligand]
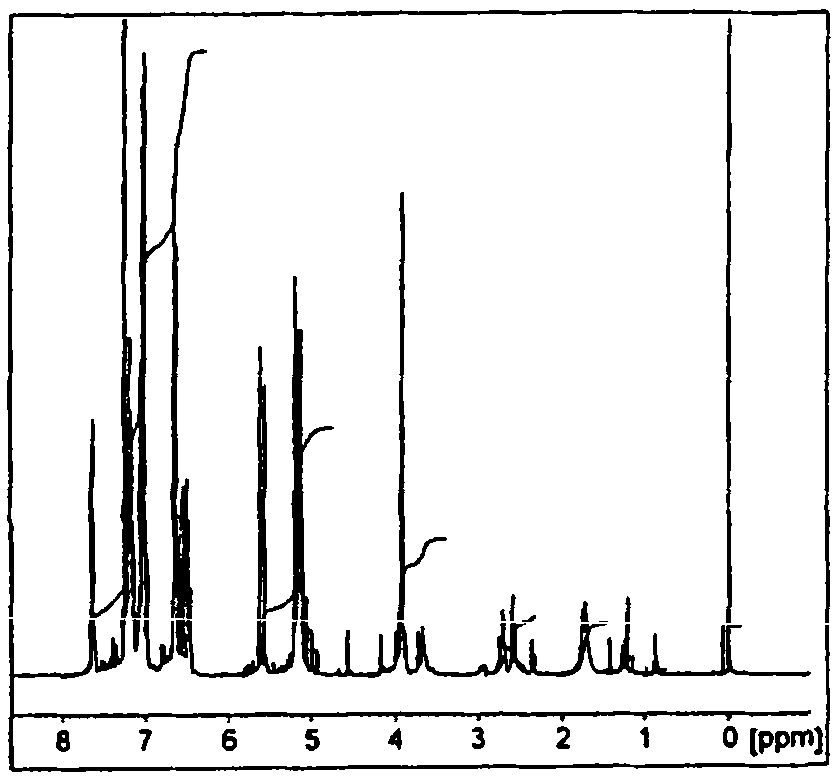
[0251] According to the synthesis of the HL-Et ligand described in J.Am.Chem.SOC.1984,106,pp4765-4772., 2-hydroxyl-1,3-diaminopropanetetraacetic acid and o-diaminobenzene were reacted, and then 4-Chloromethylstyrene was reacted to obtain bbpr-CH represented by the following chemical formula (5) with a yield of 85%. 2 St ligand. To measure the ligand's 1 H-NMR (0.05% (v / v) TMSCDCl 3 solution), the introduction of -CH was confirmed from the peak at 5-8ppm 2 St base. 1 The H-NMR diagram is shown in figure 1 .
[0252]
manufacture example 2
[0253] (Production Example 2) [Synthesis of Complex Monomer Precursor]
[0254] Measure p-vinylbenzoic acid (10.1g, 67.5mmol) and NaOH aqueous solution (10.2g, 64.1mmol) in a flask, add 140ml of water therein and stir to dissolve it, filter out insoluble components, and prepare sodium p-vinylbenzoate aqueous solution. Measure Mn(SO 4 )·5H 2 O (7.74g, 32.1mmol) and 50ml of water were stirred to dissolve. The above aqueous sodium p-vinylbenzoate solution was added thereto, followed by stirring at room temperature for 2 hours. The generated precipitate was collected by filtration, washed with water and ether, and then dried under reduced pressure to obtain a white powder of manganese p-vinylbenzoate·tetrahydrate (complex monomer precursor). The yield was 5.87 g (13.9 mmol), a 43% yield. Elemental Analysis Calcd for C 18 h 22 MnO 8 =C, 51.32; H, 5.26. Found: C, 51.63; H, 5.16.
manufacture example 3
[0255] (Manufacture example 3) [Manufacture of complex monomer]
[0256] Measure bbpr-CH in the flask 2 St (400mg, 0.372mmol), NEt (i-Pr) 2 (43.2mg, 0.335mmol), THF 54ml was added thereto and stirred to dissolve. Manganese p-vinylbenzoate·4 hydrate (313 mg, 0.744 mmol) was added thereto, followed by stirring at room temperature for 2 hours. The reaction mixture was concentrated under reduced pressure, and the precipitate generated by adding MeOH was collected by filtration, washed with water and ether, and then dried under reduced pressure to obtain Mn -vb-(bbpr-CH 2 St)-vb (complex monomer) light brown powder. Yield was 122 mg. ESI MS, m / Z 1477.4 ([M-(p-vinylbenzoic acid anion)] + ).
[0257]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com