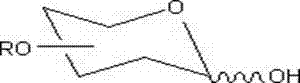
Method for removing trichloroethyl of glucoside
A technology of trichloroethyl and trichloroethyl glucoside, which is applied in the direction of chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of narrow application range, troublesome post-processing, long reaction time, etc., and achieve reaction The effect of short time, convenient post-processing and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 2,2,2-trichloroethyl-β-O-tetraacetylglucoside 80mg (0.167mmol), Zn powder 87mg (1.338 mmol) and NH 4 Cl 27mg (0.504mmol), sequentially added to 2ml of analytically pure ethanol, and then heated to reflux for trichloroethyl removal reaction, the heating temperature is 80 ° C, the reaction time is 5min, the reaction product is filtered and concentrated under reduced pressure Finally, 57.2 mg of 2,3,4,6-O-tetraacetylglucose was obtained, and the yield was 98.5%.
[0020] The product 2,3,4,6-O-tetraacetylglucose obtained from the above-mentioned examples is carried out 1 H-NMR analysis, the test data are as follows :
[0021] 1 H NMR (500MHz, CDCl 3 ): δ5.55(t,J=10Hz,0.75H), 5.47(d,J=4Hz, 0.73H), 5.26(t,J=10Hz,0.3H),5.09(t,J=10Hz,1H) ,4.86-4.92(m, 1H) ,4.75 (d,J=8Hz, 0.27H),4.22-4.30(m,2H),4.12-4.18(m,1.4H), 3.75(m,0.3H), 2.00 -2.20 (4s, 12H).
Embodiment 2
[0023] Take 2,2,2-trichloroethyl-β-O-tetraacetylmannoside 80mg (0.167mmol), Zn powder 65mg (1.002 mmol) and NH 4 Cl 27mg (0.504mmol), sequentially added to 5ml of analytically pure acetone, then heated to reflux for trichloroethyl removal reaction, the heating temperature is 60 ° C, the reaction time is 5min, the reaction product is filtered and concentrated under reduced pressure Finally, 56.9 mg of 2,3,4,6-O-tetraacetylmannose was obtained, and the yield was 90%.
[0024] The product 2,3,4,6-O-tetraacetylmannose obtained from the above-mentioned examples was carried out 1 H-NMR analysis, the test data are as follows :
[0025] 1 H NMR (500MHz, CDCl 3 ): δ5.47(dd,J=10,3Hz,1H),5.29(m,3H), 4.25(m,2H), 4.14(d,J=10H,1H) ,2.0-2.18(4s,12H) .
Embodiment 3
[0027] Take 2,2,2-trichloroethyl-β-O-tetrabenzylgalactoside 60mg (0.089mmol), Zn powder 46mg (0.714mmol) and NH 4 Cl 38.1mg (0.712mmol) was added to 2ml of analytically pure ethanol in turn, and then heated to reflux for trichloroethyl removal reaction. The heating temperature was 50°C, and the reaction time was 1.5h. The reaction product was filtered and reduced After concentrated under reduced pressure, 49.2 mg of 2,3,4,6-O-tetrabenzylgalactose was obtained, and the yield was 95.0%.
[0028] The product 2,3,4,6-O-tetrabenzylgalactose obtained from the above-mentioned examples is carried out 1 H-NMR analysis, the test data are as follows :
[0029] 1 HNMR (500MHz, CDCl 3 ): δ7.35(m,30H),5.28(t,0.93H), 5.0~4.4(m,12.3H),415(t,0.88H),4.04(dd,0.91H),3.89(m,1.45 H), 3.76(m, 0.55H), 3.6~3.5(m, 4H), 3.11(d, 0.5H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap