Methylprednisolone aceponate new crystal form and preparation method thereof
A technology of methylprednisolone acetonide and crystal, applied in directions such as pharmaceutical formulations, medical preparations containing active ingredients, steroids, etc., can solve problems such as no reports on the crystal form of methylprednisolone acetonide.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
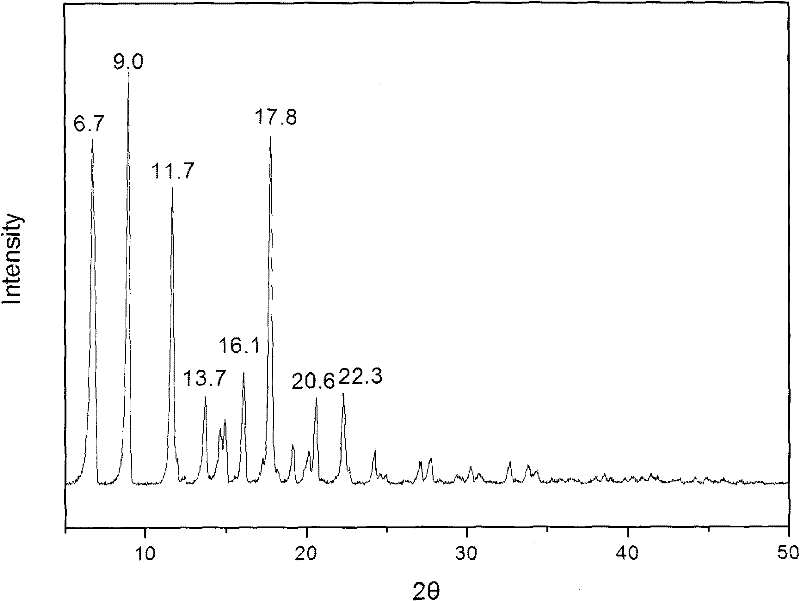
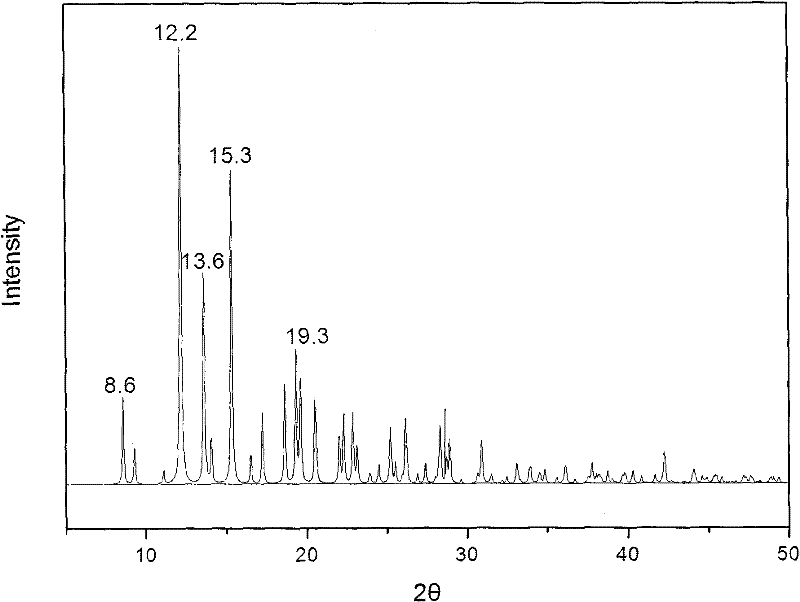
[0025] Get 1kg of methylprednisolone acetate and completely dissolve it in 10L of tetrahydrofuran, then add a dissolution agent mixed with 5L of water and 5L of ethanol to the tetrahydrofuran in which methylprednisolone acetate has been dissolved, add it slowly while adding the dissolution agent While oscillating, until the turbidity that appeared when the eluent was just added disappears, evaporate under reduced pressure after the addition, and cool down, filter, and dry after crystals appear. The water content of the dried crystal was measured by the Karl Fischer method, and it was confirmed to be methylprednisolone acetate monohydrate. X-ray powder diffraction measurement, the measured characteristic peak positions are 2θ=8.6°, 12.2°, 13.6°, 15.3°, 19.3°, such as figure 2 shown.
Embodiment 2
[0027] Get 1kg of methylprednisolone acetate and dissolve it completely in 40L of acetonitrile, then add 4L of water and 20L of normal hexane as a dissolution agent to the acetonitrile in which methylprednisolone acetate has been dissolved, add slowly while adding the dissolution agent While oscillating, until the turbidity that appeared when the eluent was just added disappears, evaporate under reduced pressure after the addition, and cool down, filter, and dry after crystals appear. The water content of the dried crystal was measured by the Karl Fischer method, and it was confirmed to be methylprednisolone acetate monohydrate. X-ray powder diffraction measurement, the measured characteristic peak positions are 2θ=8.6°, 12.2°, 13.6°, 15.3°, 19.3°.
Embodiment 3
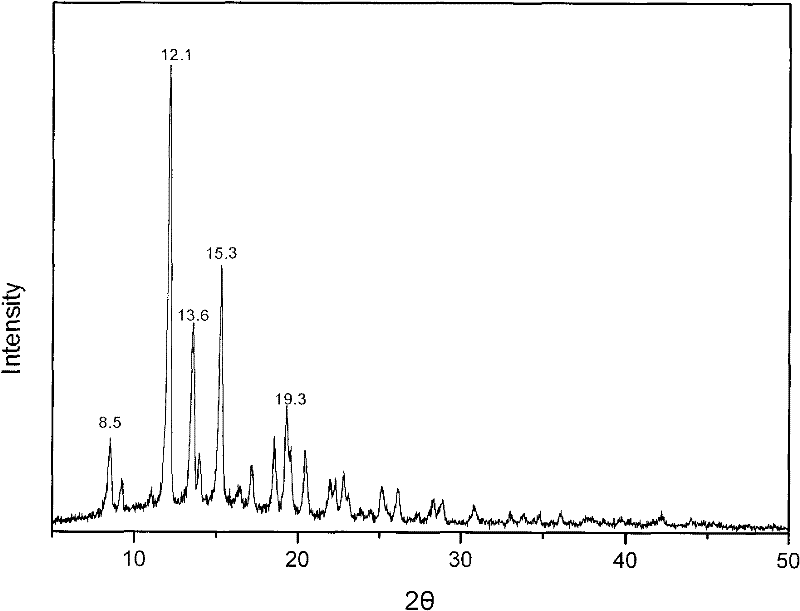
[0029] Add 0.2 g of methylprednisolone acetate into a mixed solution of 6 mL of tetrahydrofuran, 1 mL of water, and 2 mL of methanol, and stir until completely dissolved. The solution was transferred to a small beaker, sealed with parafilm, and a few small holes were punched on the film to facilitate solvent evaporation. After standing at room temperature for several days, crystallization was obtained. The water content of the dried crystal was measured by the Karl Fischer method, and it was confirmed to be methylprednisolone acetate monohydrate. X-ray powder diffraction measurement, the measured characteristic peak positions are 2θ=8.5°, 12.1°, 13.6°, 15.3°, 19.3°, such as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com