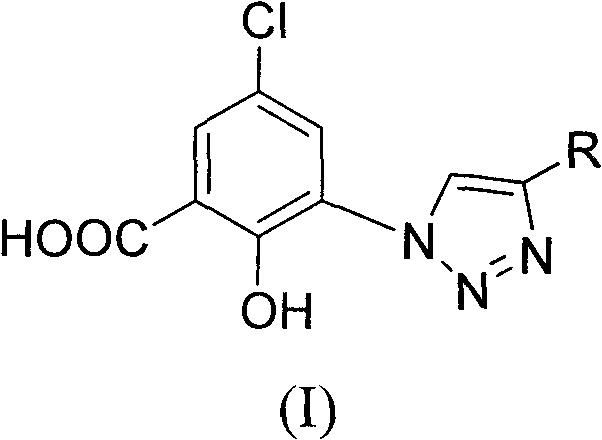
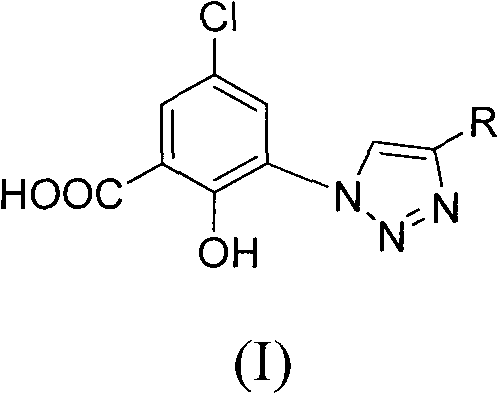
5-chlorol-2-hydroxyl-3-(4-substituted-1h-1,2,3-triazole) benzoic acid compound as well as preparation method and application thereof
A benzoic acid, -CH2XCH2R2 technology, which is applied in the field of drug synthesis and achieves the effects of simple steps, mild reaction conditions and obvious integrase inhibitory activity
Inactive Publication Date: 2010-08-25
BEIJING UNIV OF TECH
View PDF0 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Through the analysis and optimization of the structure-activity relationship of these compounds, on the basis of bioisosteric rational drug design, 5-chloro-2-hydroxy-3-(4-substituted-1H-1,2,3-tri azoles) benzoic acid compounds as HIV-1 integrase inhibitors, there is no report at home and abroad at present
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a 5-chlorol-2-hydroxyl-3-(4-substituted-1H-1,2,3-triazole) benzoic acid compound expressed in the formula (I) as well as a preparation method and application thereof. The definition of R is shown as the specification. In the preparation method, 5-chlorolsalicylic acid used as a raw material is nitrified and reduced, is subjected to azidation and reacts with end alkyne to obtain the corresponding 5-chlorol-2-hydroxyl-3-(4-substituted-1H-1,2,3-triazole) benzoic acid compound. The compound has the inhibiting function on HIV-1 integrase, and the IC50 of part of the compound reaches 1.6 mircograms / mL, therefore, the compound is a stronger HIV-1 integrase inhibiting agent and is expected to be developed into a new HIV virus resistant medicament.
Description
technical field The invention belongs to the field of drug synthesis, and specifically relates to 5-chloro-2-hydroxyl-3-(4-substituted-1H-1,2,3-triazole) benzoic acid compounds, their preparation method and their anti-HIV-1 Drug application. Background technique AIDS is an infectious disease caused by human immunodeficiency virus (HIV) infection, and it is one of the most harmful and devastating infectious diseases in human history. The research on the pathogenic mechanism of AIDS can find that in the process of HIV replication, three enzymes, namely reverse transcriptase, integrase and protease, participate successively. Since the FDA approved Zidovudine (AZT) for clinical use in 1987, up to 2005 the FDA has approved a total of 27 new drugs for the treatment of AIDS. Mainly divided into nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors and fusion inhibitors. Unfortunately, currently clinically used medicine...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D249/06A61K31/4192A61P31/18
Inventor 曾程初杨城文刘称福胡利明盛望
Owner BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com