4-substituted anilino-podophyllotoxine derivative and application
An epipodophyllotoxin and anilino-based technology, applied in the field of 4-substituted anilino epipodophyllotoxin derivatives and their applications, can solve the problems of bone marrow suppression, poor water solubility, and easy drug resistance
Inactive Publication Date: 2010-09-01
ZHEJIANG UNIV
View PDF1 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Although the above two compounds have been used clinically for many years, there are still problems such as poor water solubility, bone marrow suppression, and easy drug resistance, which limit their application.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
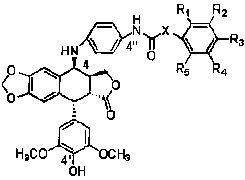
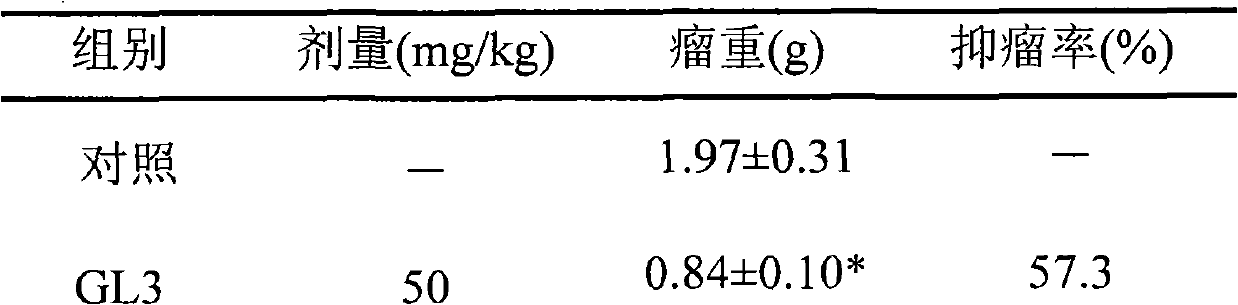
The invention provides a 4-substituted anilino-podophyllotoxine derivative which refers to a 4'-O-demethyl-4-deoxy-4-substituted anilino-podophyllotoxine derivative. A series of compounds with anti-cancer activity is synthetized by taking the traditional drug etoposide as a lead compound by structural transformation; primary in vitro screening and in vivo antitumor tests show that the compounds have better anti-tumor activity, wherein when the compound GL3 improves the anti-tumor action, the toxicity of the compound GL3 is obviously reduced, and thereby, the compound GL3 can be used for preparing anti-tumor drugs which has higher antitumor activity and lower toxicity and has effect on multi-drug drug resistant tumors. The structural general formula of the 4-substituted anilino-podophyllotoxine derivative is shown in the specification.
Description
technical field The invention belongs to the preparation and use of compounds, and mainly relates to the preparation of 4'-O-desmethyl-4-deoxy-4-substituted anilino epipodophyllotoxin derivatives and their application in the preparation of antitumor drugs. Background technique Etoposide and teniposide are currently clinically used antineoplastic drugs, both of which are semi-synthetic derivatives of 4'-O-desmethyl epipodophyllotoxin. 4'-O-desmethyl epipodophyllotoxin derivatives can destroy the structure and function of DNA by inhibiting the activity of DNA topoisomerase II, thereby exerting an anti-tumor effect. Although the above two compounds have been used clinically for many years, they still have problems such as poor water solubility, bone marrow suppression, and easy drug resistance, which limit their application. Therefore, in order to overcome the above shortcomings and find more efficient and low-toxic podophyllotoxin compounds, researchers have carried out a se...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D493/04A61K31/365A61P35/00
Inventor 胡永洲汪丽杨波何俏军
Owner ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com