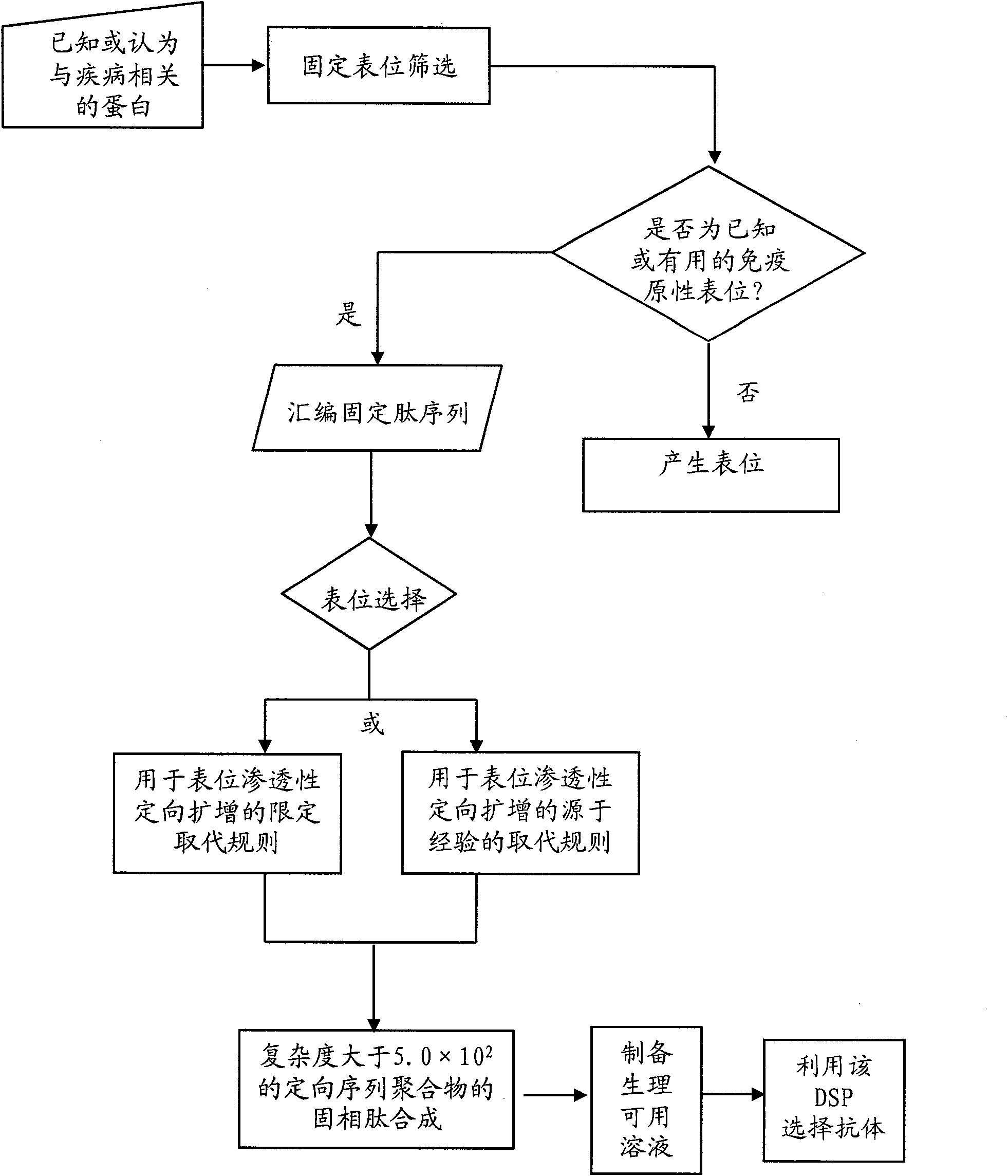
Methods for the directed expansion of epitopes for use as antibody ligands
An antibody and epitope technology, which can be used in the preparation methods of peptides, pharmaceutical formulations, antibody medical components, etc., and can solve problems such as logistics and cost obstacles and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 Preparation of DSP Compositions Based on Fictitious Peptides
[0136] For ease of understanding, and by way of example, the table in Figure 6 describes and represents the preparation of DSP compositions from two imaginary peptide sequences representing a known epitope. In this example, each box consists of 5 amino acids, (y 1 In x1, x2, x3, x4, x5=THMCE, and y 2 in PWKNA). The THMCE is defined to have input ratios of a=7, b=1, c=1, d=1, e=10. PWKNA is defined to have input ratios a=1, b=3, c=3, d=3, e=20. For synthesis, the group of amino acids occupying each amino acid position of each peptide is identified by the preferred amino acid substitution method shown in Figure 4 and described in Kosiol et al., J. Theoretical Biol. 228:97-106 , 2004 (or an equivalent method that systematically changes amino acids is less preferred), and the total ratio of amino acids occupying each position in the resulting overall DSP composition is given above. Each box, yi an...
Embodiment 2
[0140] Example 2 Preparation of Gp100 (amino acid residues 154-162) as the DSP composition of the source peptide
[0141] Figures 7A-B show examples of DSP synthesis rules using Gp100 (amino acid residues 154-162) as the source peptide. The method and rules for defining the amino acid species at each position of the generated peptides are described in Example 1 above. As in Example 1, the DSP composition was synthesized by a solid-phase peptide synthesis method.
Embodiment 3
[0142] Embodiment 3 prepares the DSP composition of HLA peptide as source peptide
[0143] Figures 8A-B show examples of DSP synthesis rules using HLA-derived peptides and HLA-mimetic source peptides as source peptides. The method and rules for defining the amino acid species at each position of the generated peptides are described in Example 1 above. As in Example 1, the DSP composition was synthesized by a solid-phase peptide synthesis method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com