R-3-hydroxybutyrate methyl ester biosynthesis preparation method
A technology for methyl hydroxybutyrate, biosynthesis, applied in microorganism-based methods, biochemical equipment and methods, carboxylate preparation, etc., can solve problems such as low conversion rate and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be described in detail below by describing the preferred embodiments of the present invention. The following description does not limit the invention.
[0032] (1) Microbial fermentation
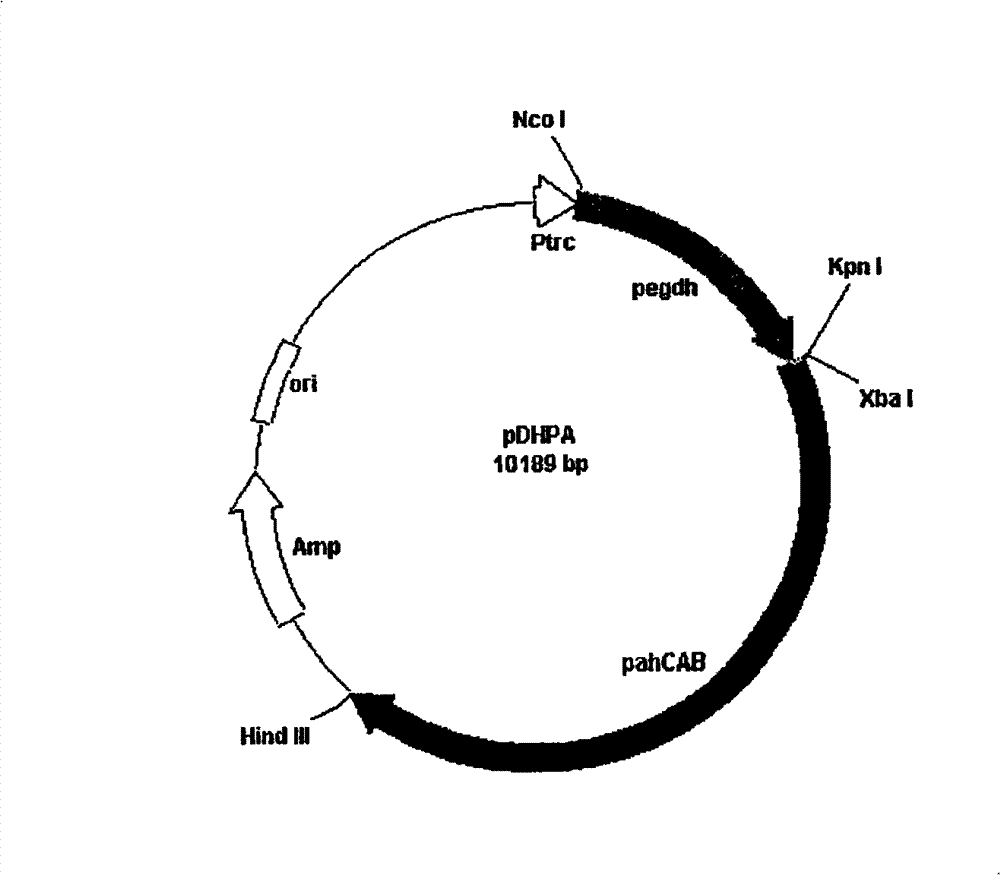
[0033] Recombinant strains: β-ketothiolase (phaA), acetoacetyl-CoA reductase (phaB), and PHA synthase (phaC) for the biosynthesis of R-3-hydroxybutyric acid were cloned by molecular biology methods; Engineering technology constructs expression vectors of the above biosynthetic enzymes and introduces them into microorganisms capable of synthesizing R-3-hydroxybutyric acid to obtain recombinant strains;
[0034] Bacterial growth medium (LB): yeast powder 5g / L, peptone 10g / L, NaCl 10g / L, pH7.2.
[0035] Fermentation medium: 4.5g / L KH 2 PO 3 , 3.6g / L (NH 4 ) 2 SO 4 , 1.23g / L MgSO 4 , 2.04g / LNH 4 CI, 1g / L citric acid, 100mg / L ampicillin and 1ml / L trace elements ((g) in 1mol / L HCl: 0.05FeSO 4 ·7H 2 O, 0.011ZnSO 4 ·7H 2 O, 0.0025MnSO 2 4H 2 O, 0.005C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com