Method of recycling useful metal
一种再循环、金属的技术,应用在回收利用技术、工艺效率的提高、固体废物的清除等方向,能够解决废弃物不能同时处理、没有具体地公开等问题,达到提高溶解速度、低能耗的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
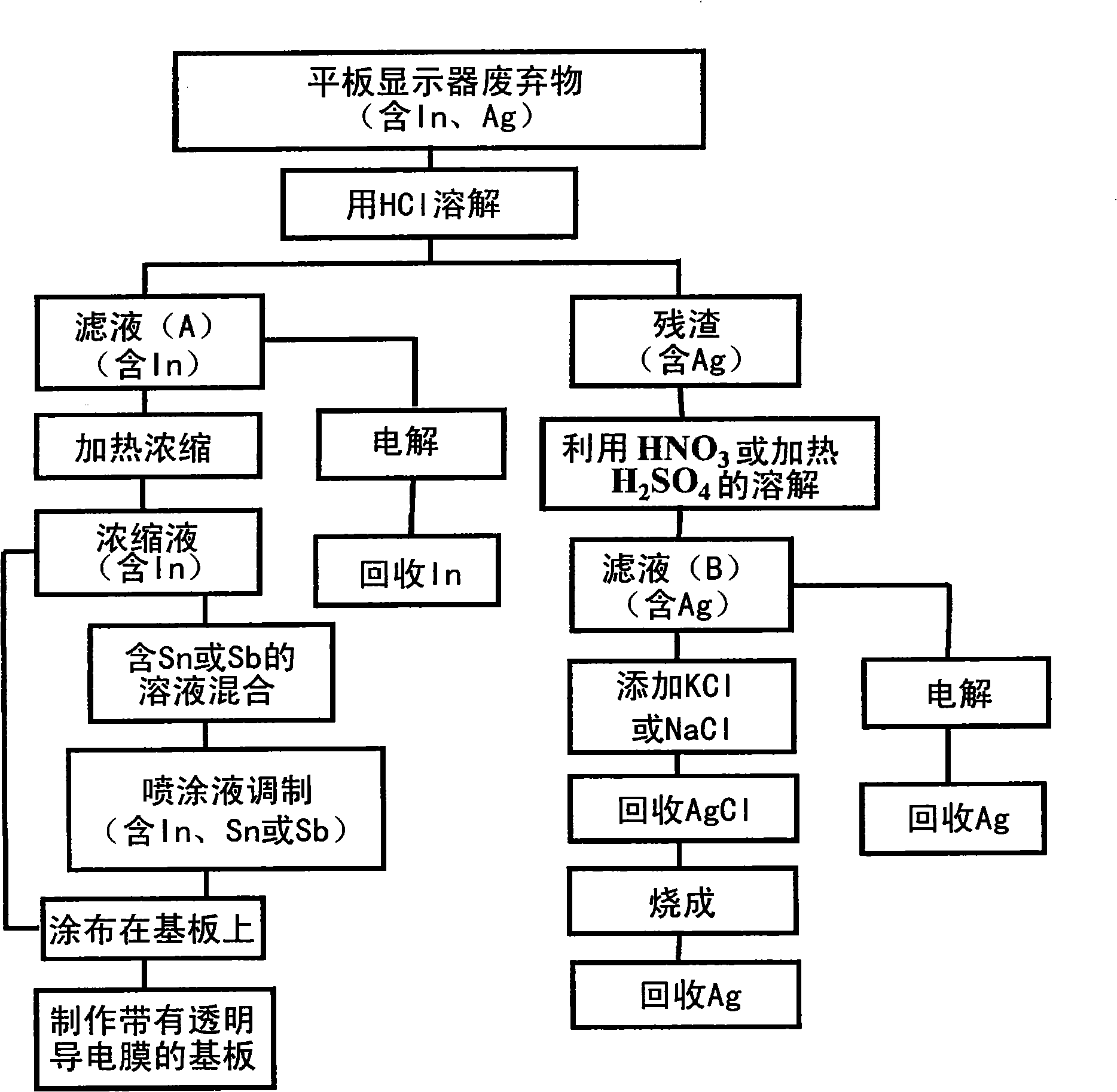
[0137] Embodiment 1. Recover indium, tin ( figure 2 、25)
[0138] This embodiment mainly relates to claims 1, 2, 3 and the like.
[0139] 2 time)>
[0140] In this embodiment, considering that the transparent electrode (ITO) contains In 2 o 3 and tin oxide (SnO 2 ), investigated In 2 o 3 and SnO 2 Solubility in hydrofluoric acid (HF) aqueous solution.
[0141] First, for indium oxide (In 2 o 3 ) powder dissolved in hydrofluoric acid (HF) for semiconductors was investigated for solubility. Pure water was added to the HF aqueous solution with a concentration of 49% by weight, the HF concentration was adjusted in the range of 10% to 49% by weight, and the In with a purity of 99.99% 2 o 3 The powder was dissolved in 100g of HF aqueous solution, Figure 4 shows the results of investigations on the solubility at a temperature of 24°C. in 2 o 3 In the processing of membranes, HCl and HNO are generally used 3 Aqua regia is the main component, but it is found to be ...
Embodiment approach 2
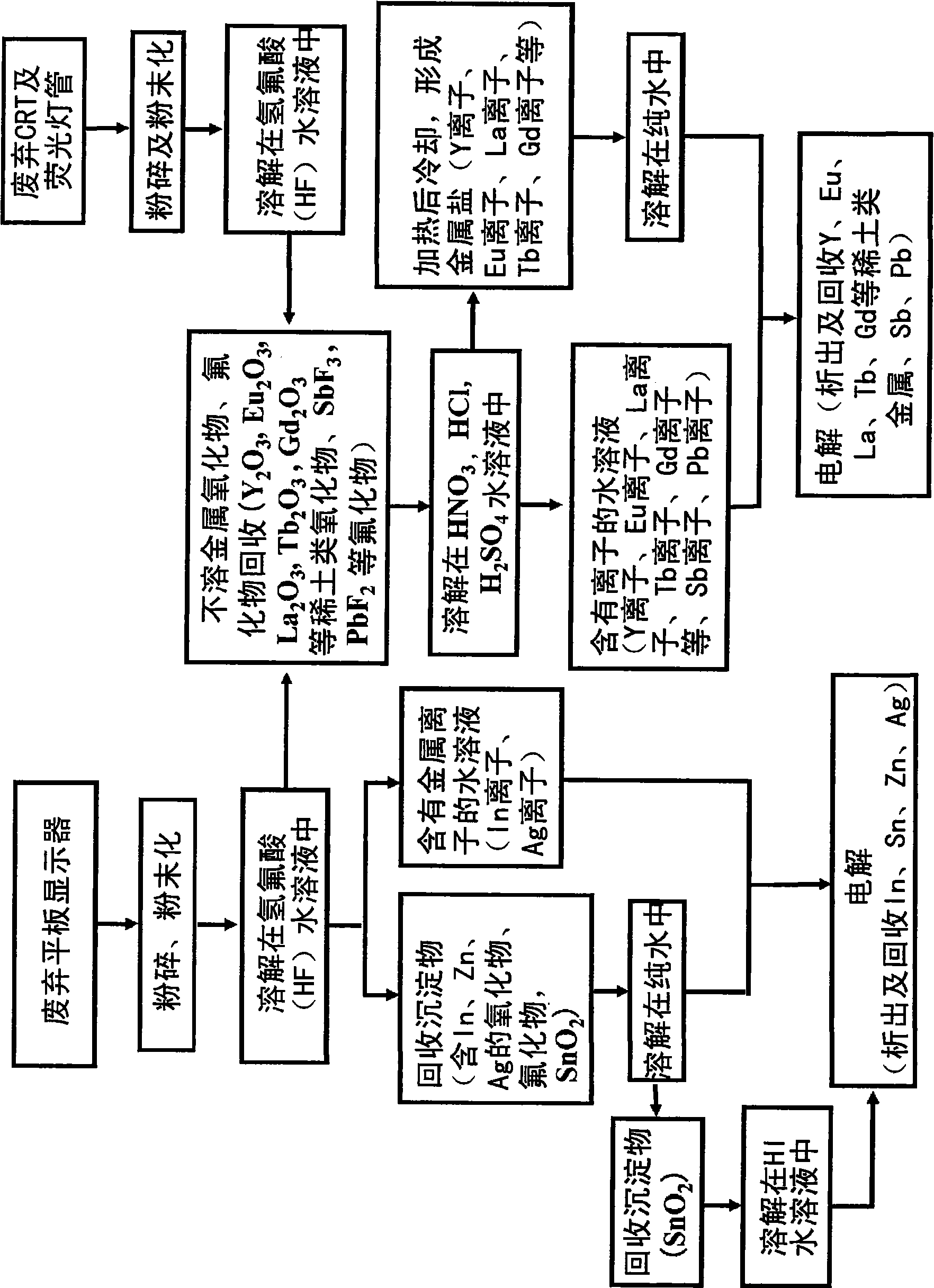
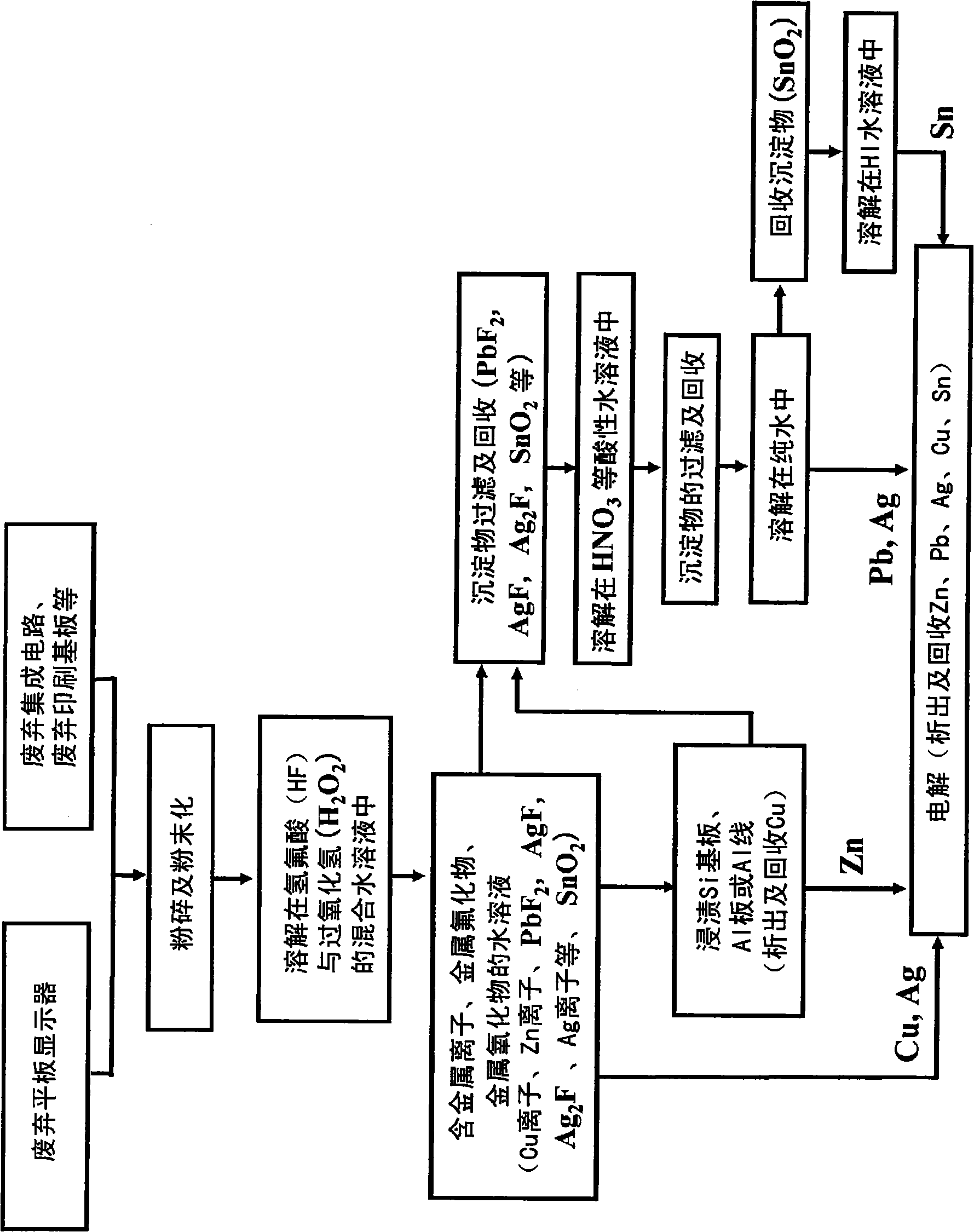
[0161] Embodiment 2. Recovery of zinc from zinc oxide for transparent electrodes
[0162] This embodiment mainly relates to claims 1, 2, 3, etc., and describes a method of recovering (Zn) from zinc oxide (ZnO) studied as a metal substitute for indium for transparent electrodes.
[0163] 2 time)>
[0164] First, the solubility of ZnO in HF aqueous solution was investigated. Add pure water to a 49% by weight hydrofluoric acid (HF) aqueous solution for semiconductors to adjust the HF concentration, Figure 10 24 shows the results of the solubility survey when ZnO (purity: 99%) powder was dissolved in 100 g of HF aqueous solution at a temperature of 24°C. from Figure 10 It was found that ZnO is soluble in HF aqueous solution. As the HF concentration increases, the solubility of ZnO also increases, and reaches the maximum at HF concentration of 40% by weight. When the HF concentration is further increased, the solubility tends to decrease. That is, from Figure 10 It was ...
Embodiment approach 3
[0173] Embodiment 3. Recovery of rare earth metals such as yttrium, europium, lanthanum, and terbium from fluorescent materials
[0174] This embodiment mainly relates to claims 4, 6, 7 and the like.
[0175]
[0176] In the present embodiment, first, yttrium oxide (Y 2 o 3 ), europium oxide (Eu 2 o 3 ), lanthanum oxide (La 2 o 3 ), terbium oxide (Tb 4 o 7 ) Solubility in various acidic aqueous solutions.
[0177] As the acidic aqueous solution, a 49% HF aqueous solution for semiconductors, a 10% nitric acid (HNO 3 ) aqueous solution, concentration of 10% sulfuric acid (H 2 SO 4 ) aqueous solution, a concentration of 10% hydrochloric acid (HCl) aqueous solution, at a temperature of 24 ° C, the Y with a purity of 99.99% 2 o 3 Powder, 99.9% Eu 2 o 3 Powder, La with a purity of 98.0% 2 o 3 Powder, Tb with a purity of 99.95% 4 o 7 See Table 1 for the solubility when the powder is dissolved in each 100 g of aqueous solution.
[0178] Table 1
[0179]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com