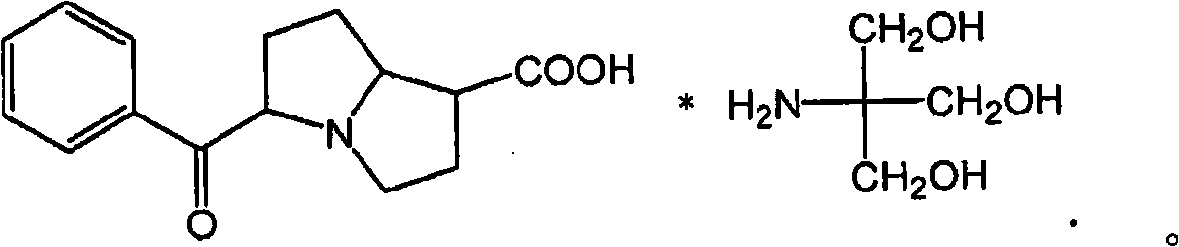Ophthalmic nsaids as adjuvants
An ophthalmic and adjuvant technology, applied to medical preparations containing active ingredients, anti-inflammatory agents, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Example 1: Combination Approach Using NSAIDs and Antiangiogenic Therapy in the Treatment of Ocular Angiogenic Diseases
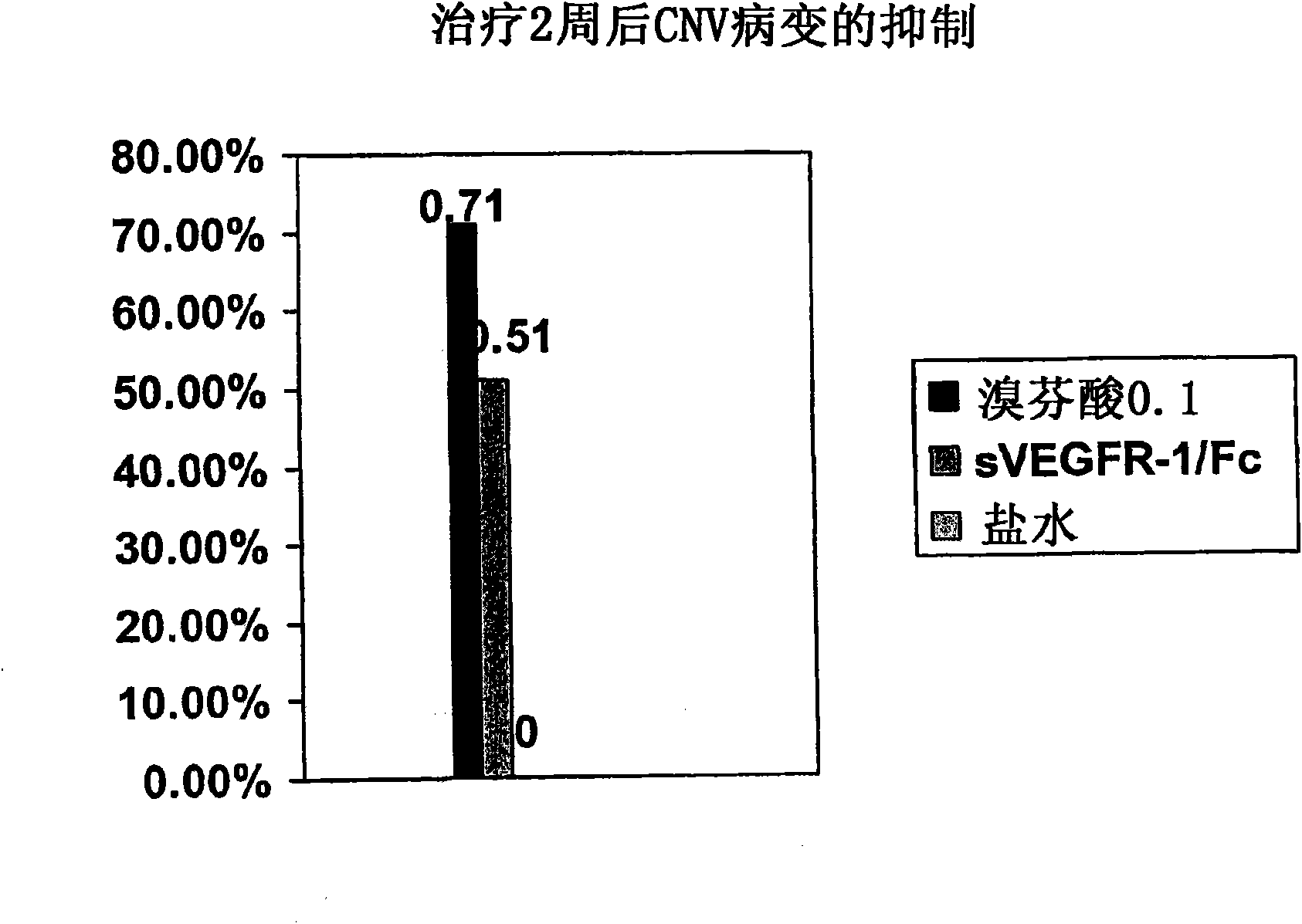
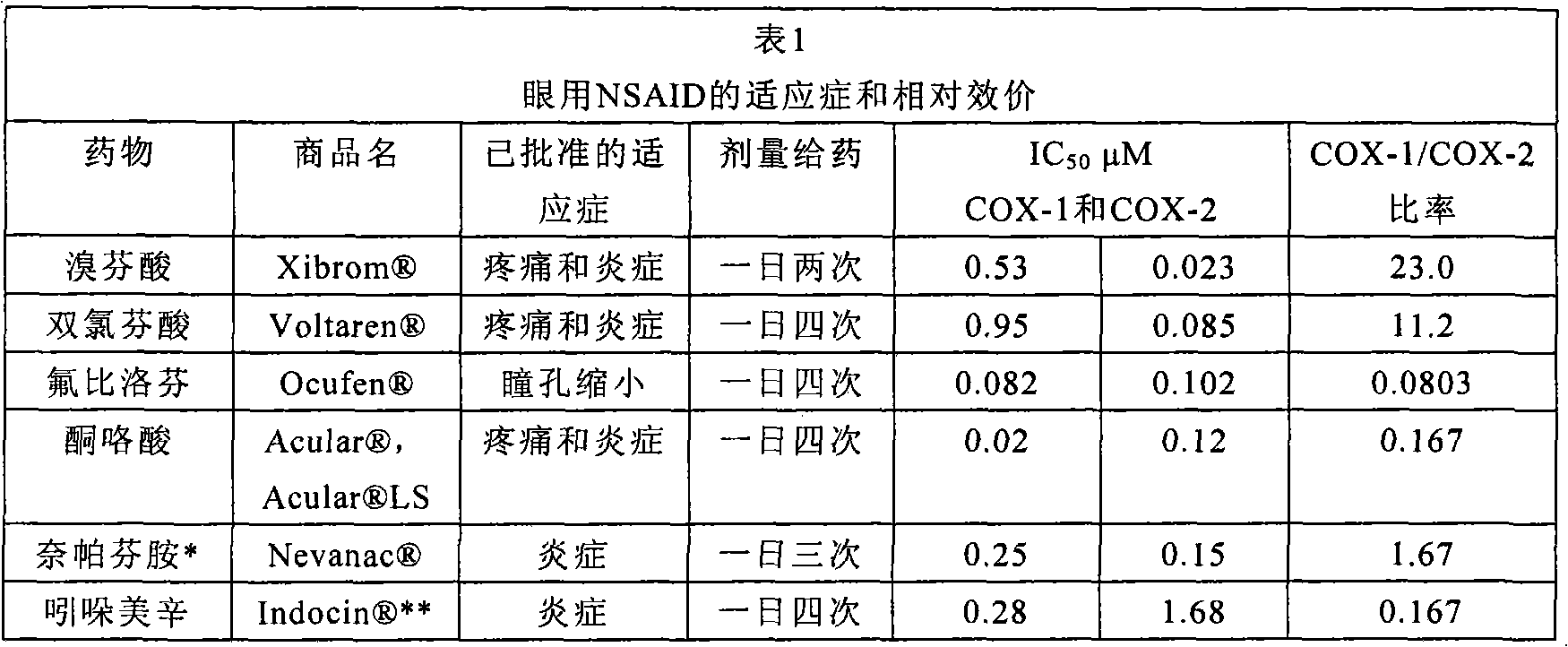
[0169] Recent data in a mouse model of retinal neovascular disease showed a beneficial effect of bromfenac ophthalmic solution when administered topically to mice with laser-induced angiomatous retina. like figure 1 As shown in , the antiangiogenic effect produced by bromfenac was greater than that obtained using intravitreal administration of soluble VEGF receptors. figure 1 showed inhibition of choroidal neovascularization (CNV) lesions after 2 weeks of topical administration of 0.1% bromfenac ophthalmic solution (BF) to mice with CNV induced by laser photocoagulation; and 0.1% BF vs. Effects of Vascular Endothelial Growth Factor (VEGF) Neutralizing Protein, Recombinant Murine Soluble Receptor 1 / Fc Chimeric Protein (sVEGFR-1 / Fc). This study showed that the area of choroidal neovascularization was reduced by 71% with bromfenac treatment compare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com