Vibrio cholerae typing and virulence gene detection kit and detection method
A detection kit and Vibrio cholerae technology, which is applied in biochemical equipment and methods, material stimulation analysis, microbial measurement/inspection, etc., can solve the problems of small flux, long detection time, and high condition requirements, and achieve throughput Large, easy-to-operate, simple-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Vibrio cholerae typing and virulence gene detection kit and its use
[0049] 1. Prepare a kit comprising the following components:
[0050] DNA extraction solution (1.2 mL / tube) 1 tube: composed of 40 mM NaOH, 20 mM Tris-HCl (pH8.8), 5% TritonX-100, 0.1 mM EDTA (pH8.0).
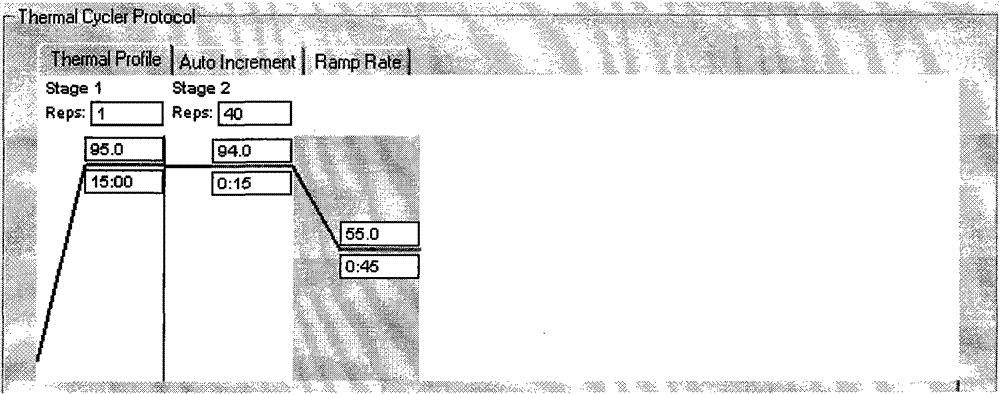
[0051] PCR reaction solution (650μl / tube) 1 tube: PCR reaction buffer consists of Tris-HCl (50mmol / L, pH8.5), MgCl 2 (8mmol / L), KCl (250mmol / L) and dNTPs (25mmol / L); The four Vibrio cholerae oligonucleotide probe sequences used for fluorescent PCR amplification are shown in the sequence table SEQ.ID.NO9-12 respectively , the two ends of the SEQ ID NO9 probe are respectively combined with the fluorescence generating group FAM and the fluorescence quenching group BHQ1, and the two ends of the SEQ ID NO10 probe are respectively combined with the fluorescence generation group CY5 and the fluorescence quenching group BHQ2, SEQ The two ends of the ID NO11 probe are respectively combined with a fluorescent ...
Embodiment 2
[0080] Application of Vibrio cholerae typing and virulence gene detection kit to detect clinical samples
[0081] Select 2 cases to be detected as Vibrio cholerae negative through the isolation and culture method, and 2 cases are detected to be Vibrio cholerae O139-positive specimens through the isolation and culture method, and the nucleic acid extraction, PCR amplification and result analysis of the samples are carried out with reference to Example 1. , Detection of positive quality control products.
[0082] Interpretation of test results:
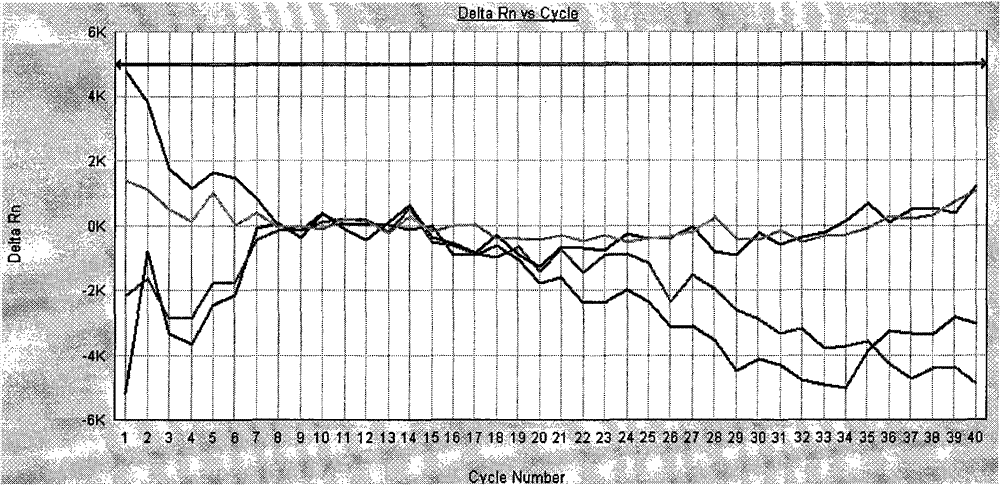
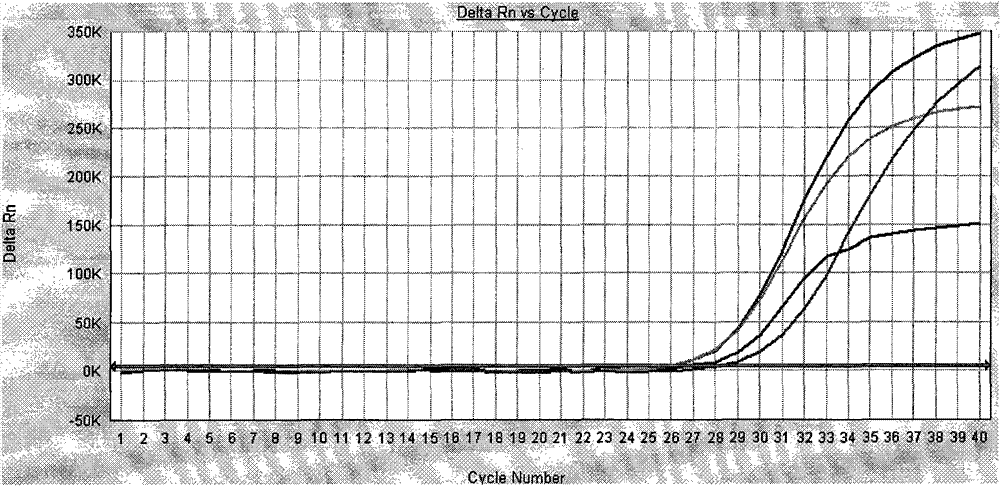
[0083] The amplification curve of the negative quality control product is not S-shaped curve, and the Ct value is greater than 37, indicating that the amplification of Vibrio cholerae nucleic acid in the negative quality control product (see attached figure 2 ). The amplification curve of the positive quality control product is an S-shaped curve, and the Ct value is less than 37, which is within the quality control range of the kit, ...
Embodiment 3
[0087] Application of Vibrio cholerae typing and virulence gene detection kit to detect clinical samples
[0088] Select 3 cases to be detected as Vibrio cholerae negative through the isolation and culture method, and 6 cases are detected to be Vibrio cholerae positive specimens through the isolation and culture method, and the nucleic acid extraction, PCR amplification and result analysis of the samples are carried out with reference to Example 1, and the negative and positive Testing of quality control products.
[0089] The detection result is explained with embodiment 2.
[0090] The amplification curves of 3 cases of Vibrio cholerae-negative specimens tested by the isolation and culture method did not show an S-shaped curve, and the Cts were all greater than 37, indicating that there was no amplification of Vibrio cholerae nucleic acid in the 3 samples (see attached Image 6 ).
[0091] 6 samples tested positive for Vibrio cholerae by isolation and culture method were t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com