Genetically modified cell of coexpression mouse membranous type interleukin 15 and retinoic acid early transcript 1 epsilon (Rae-1 epsilon) and preparation method thereof
A technology of transgenic cells and interleukins, applied in the field of immunology, can solve the problems of low content, cumbersome operation, low IKDC amplification efficiency, etc., and achieve the effects of reduced operation steps, high enrichment rate, and strong extensibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
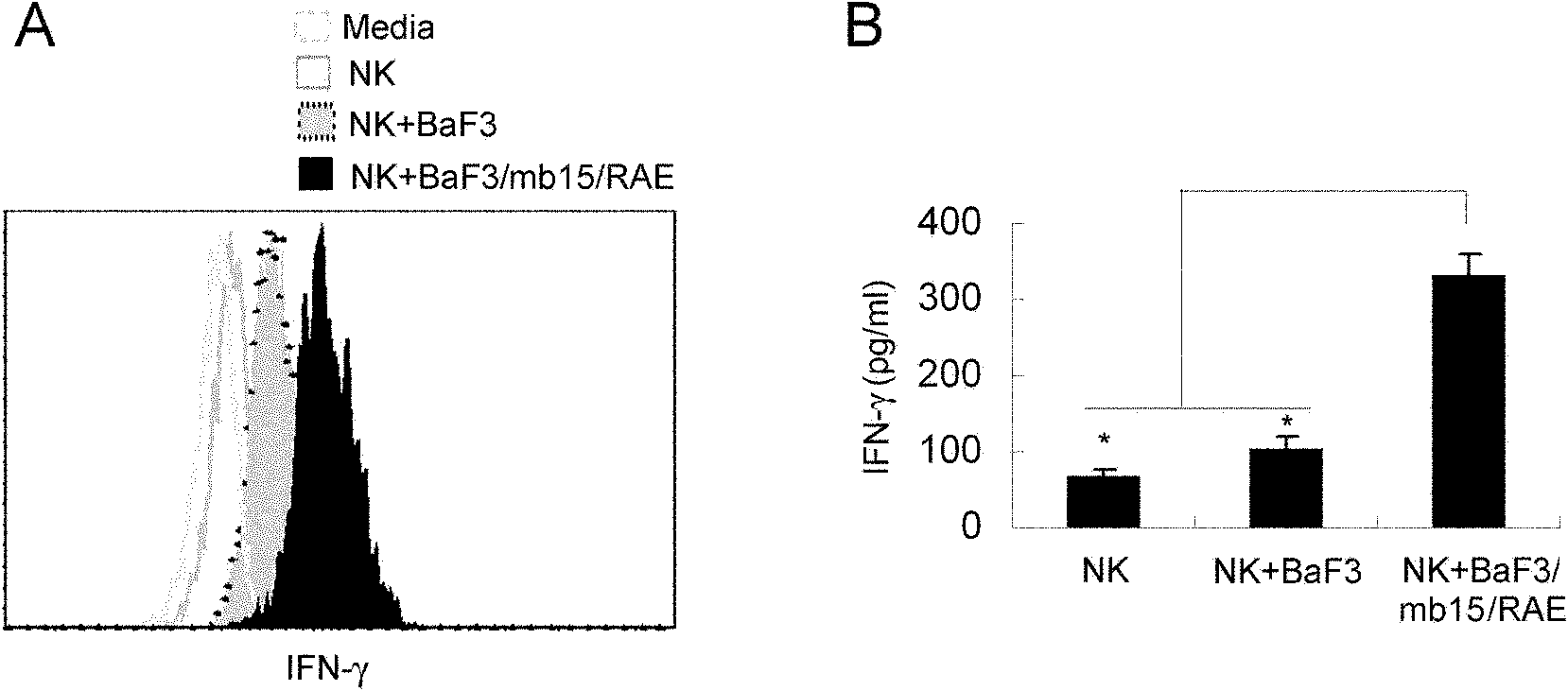
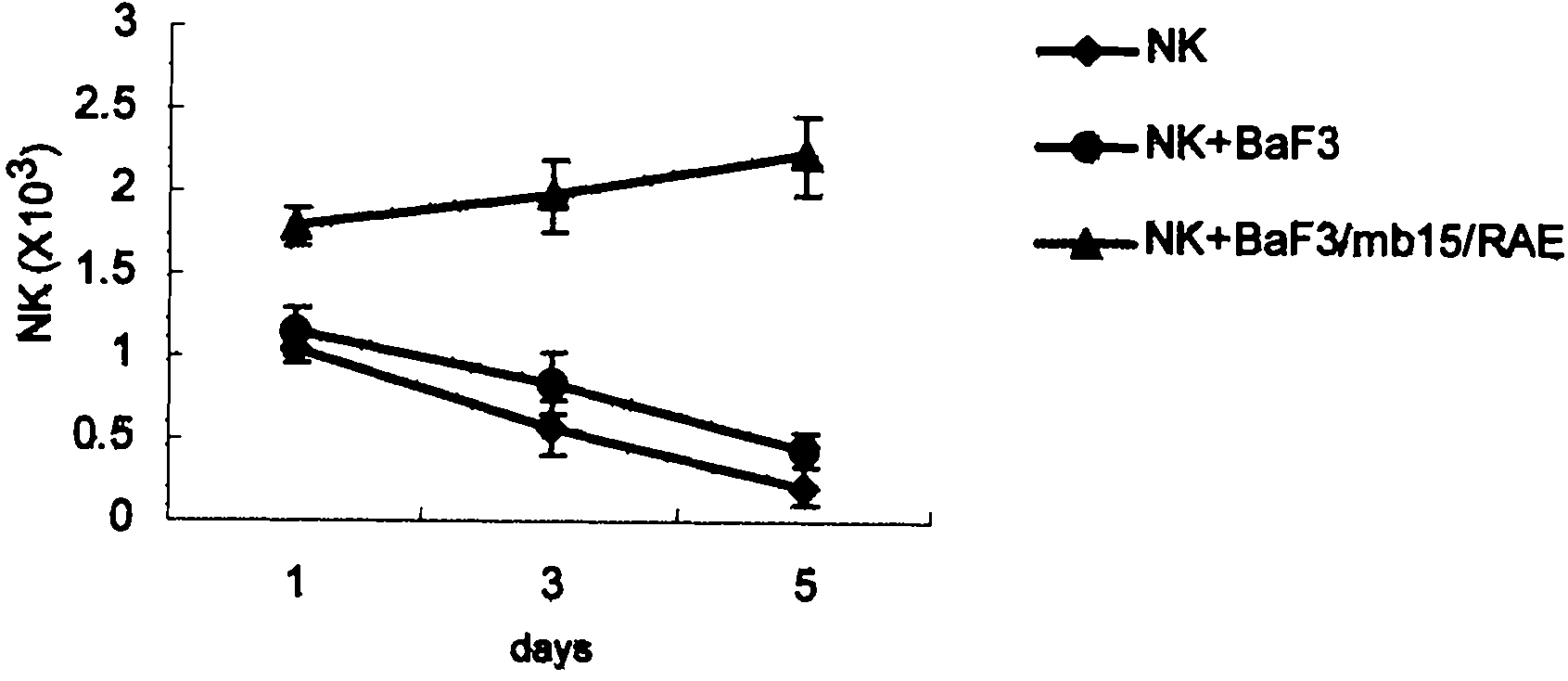
Examples
Embodiment Construction
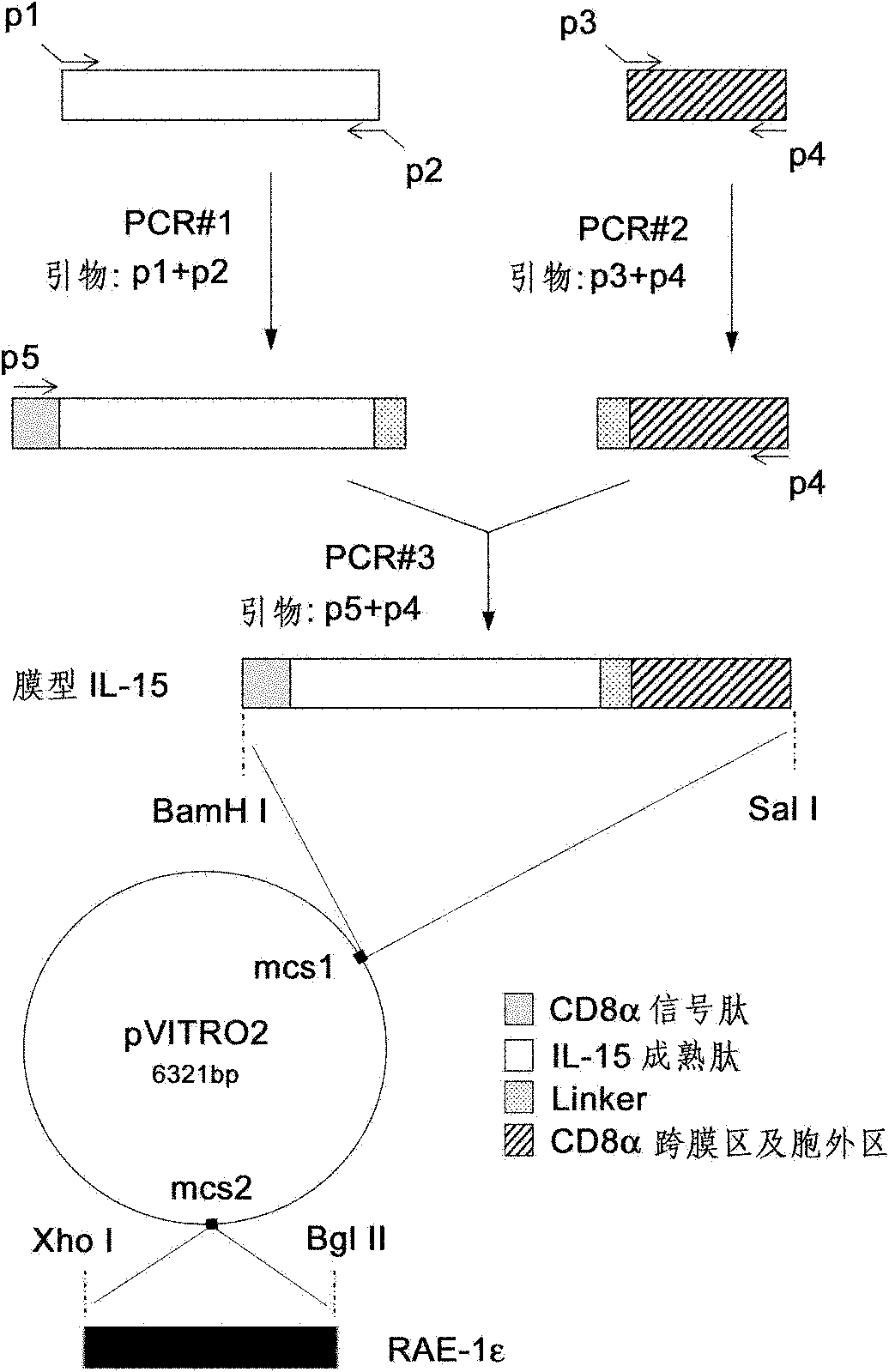
[0011] Description of raw materials:
[0012] 1. CD8α cDNA is a published sequence (NCBI sequence number: BC030679).
[0013] 2. IL-15 cDNA is the published sequence (NCBI sequence number: NM_008357).
[0014] 3. RAE-1ε cDNA is a published sequence (NCBI sequence number: FJ594067).
[0015] 4. The pVITRO2-mcs, pcDNA3.1(+) and pORF9-mIL-15 eukaryotic expression vectors are products of Invitrogene.
[0016] 5. The vector pMX-pie containing RAE-1ε cDNA was donated by Professor Lewis L Lanier, University of California, USA.
[0017] 6. pcDNA3 / RAE-1ε was constructed by our laboratory. Available to the public for 20 years. The specific construction process is as follows: the RAE-1ε gene is amplified by PCR technology, and the upstream and downstream primer sequences are as follows: 5'-CAG GGTACC ATGGCCAAGGCAGCAGTGACCAA-3' (SEQ ID NO: 8) and 5'-TAA GCGGCCGC TCACATCGCAAATGCAAATGCAAATAAT-3' (SEQ ID NO: 9), a Kpn I restriction site was introduced at the 5' end of the up...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com