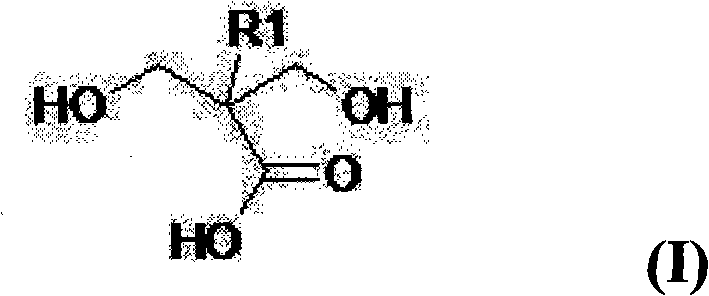
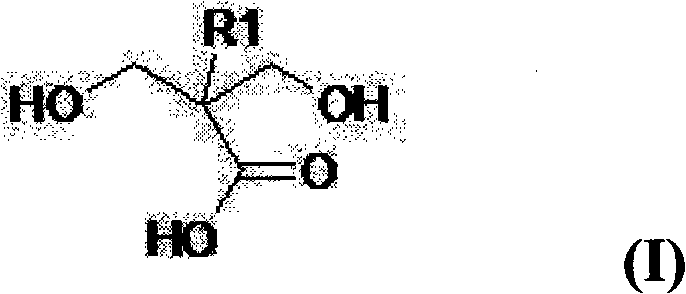
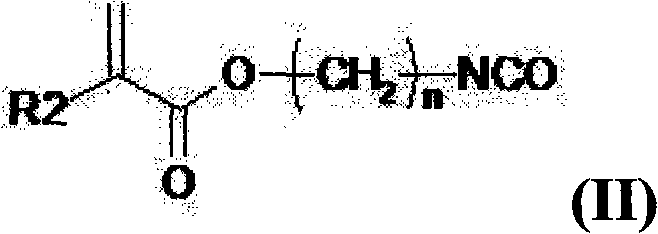Multifunctional urethane monomer, method of manufacturing the monomer and photo-sensitive resin composition including the monomer
一种酯多官能、氨基甲酸的技术,应用在氨基甲酸衍生物制备、羧酸酐制备、有机化合物的制备等方向,能够解决增加组合物粘度、光聚合引发剂价高等问题,达到好耐化学性和耐热性、优良感光性、高显影宽容度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The preparation method of alkali-soluble resin can be prepared according to the following two steps:
[0059] In the first step, monomers containing acid functional groups are copolymerized with aromatic and aliphatic vinyl monomers suitable for copolymerization to obtain alkali-soluble linear copolymers. Preferably, the aromatic and aliphatic vinyl monomers may be selected from those monomers that improve film strength. The monomer that can improve film strength may be selected from compounds having an aromatic ring.
[0060] The reaction may be carried out using a method selected from a variety of polymerization methods known in the art: free radical polymerization, cationic polymerization, anionic polymerization, polycondensation, and the like. In view of the ease of preparation and the economical effect of preparation, radical polymerization is most preferably used.
[0061] For example, it can be produced by mixing monomers with a polymerization solvent, heating ...
specific example
[0063] The free radical polymerization initiator may be selected from those known in the art, and specific examples thereof include 2,2'-azobisisobutyronitrile (AIBN), 2,2'-azobis-( 2,4-dimethylvaleronitrile), 2,2'-azobis-(4-methoxy-2,4-dimethylvaleronitrile), benzoyl peroxide, lauroyl peroxide, peroxide tert-butyl oxypivalate and 1,1'-bis-(di-tert-butylperoxy)cyclohexane, etc.
[0064] The chain transfer agent is used to control the weight average molecular weight, examples of which are: n-hexyl mercaptan, n-octyl mercaptan, n-dodecyl mercaptan, t-dodecyl mercaptan, thiol Glycolate / ester, 3-mercaptopropionic acid, α-methylstyrene dimer, etc. The chain transfer agent is not limited thereto, and may be selected from those known in the art.
[0065] Examples of monomers having acid groups that can be used to prepare the linear copolymers include: (meth)acrylic acid, crotonic acid, itaconic acid, maleic acid, fumaric acid, monomethylmaleic acid, isoprene olefin sulfonic acid, ...
Embodiment 1
[0098] 35 g of 25% carbon black dispersion, 2.5 g of the urethane polyfunctional monomer prepared in Synthesis Example 1, 12.4 g of an alkali-soluble resin (solid content: 3.5 g), 2.0 g of dipentaerythritol hexaacrylate As a polymer, 1.5 g of photopolymerization initiator OXE-02, 0.5 g of 3-{4-[2,4-bis(trichloromethyl)-s-triazin-6-yl]phenylthio}propionic acid, 0.2 g of 4,4'-bis(diethylamino)benzophenone and 44.5 g of PGMEA organic solvent were mixed with each other for 3 hours to prepare a photosensitive resin composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com