Pharmaceutical gallium compositions and methods
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, active ingredients of heavy metal compounds, etc., can solve problems such as blood pressure drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] 1 to 6 equivalents of ammonium citrate are gradually added to the aqueous solution containing 1 equivalent of Ga(NO3)3.9H2O at room temperature. At this time, spontaneous crystallization quickly appeared. Agitated at room temperature to form a mixture of precipitates (fine gelatinous substances) to reach a pH of about 3-4.5. The reaction was allowed to cool to room temperature. The nitrate in the solution was stripped from the precipitated gallium citrate by filtration through Whatman 3M filter paper. Then, the gallium citrate was washed with 50% isopropanol dissolved in water and 190 alcohol alcohol in sequence and rotary evaporated under high vacuum at 60-100° C. to produce a gallium citrate preparation in fine powder form.
Embodiment 2
[0079] Before drying, the two separate batches of gallium citrate in Example 1 were mixed with standard excipients, wet granulated and compressed into two different batches of ~725 mg tablets containing 30 mg of gallium. The tablets from each of these batches contained approximately 22 μg of nitrate. For comparison, two batches of Ga(NO 3 ) 3 ·9H 2 O Prepared into 2 different batches of 750 mg tablets containing 30 mg of gallium. The tablets from each of these batches contained approximately 80 mg of nitrate.
Embodiment 3
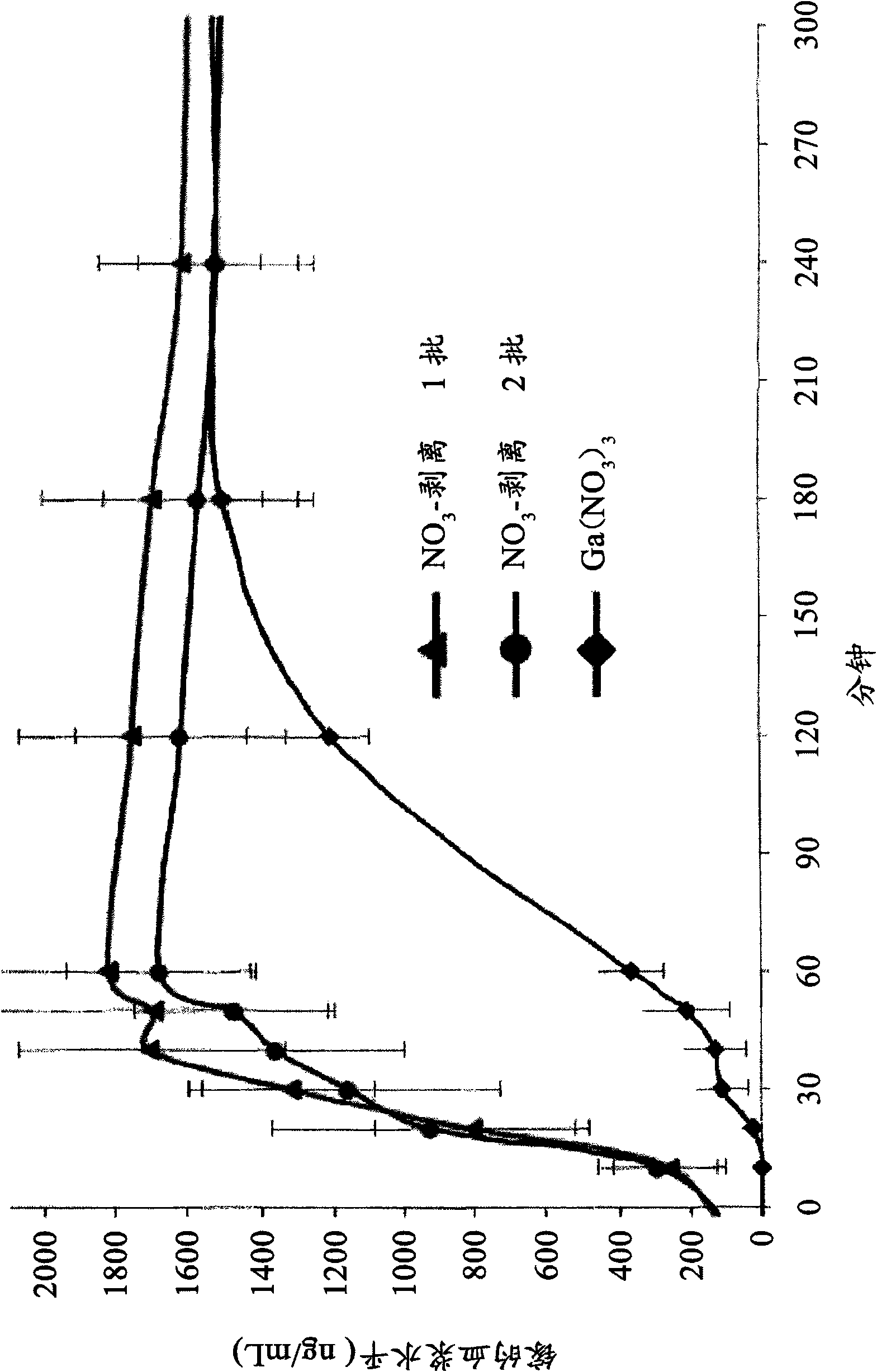
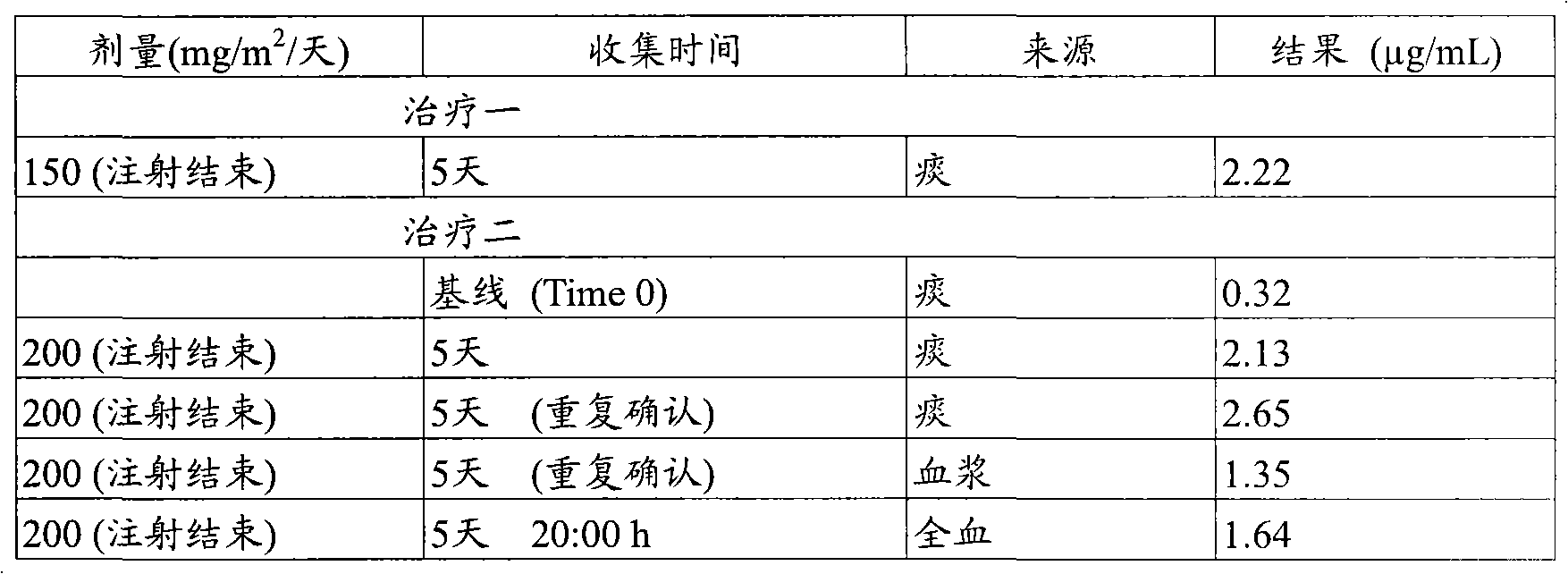
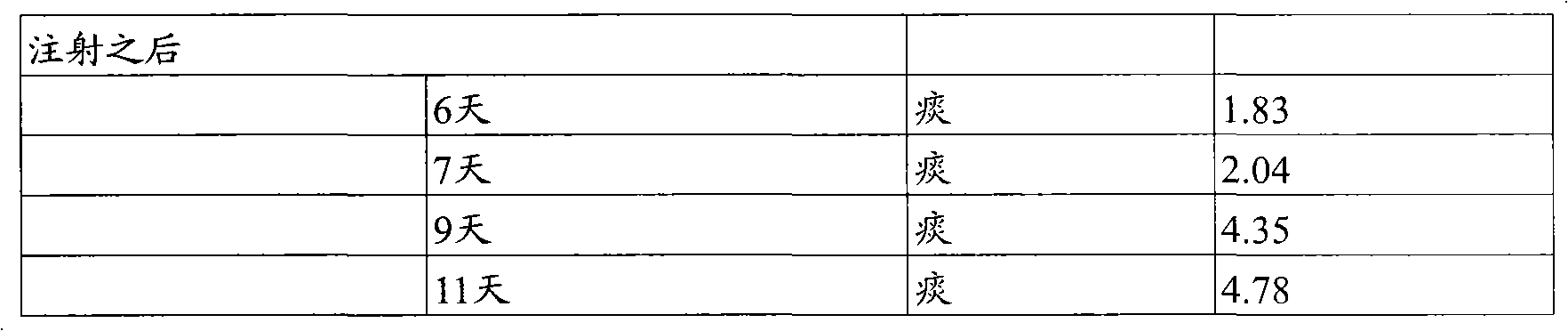
[0081] The properties of each batch of tablets in Example 2 were directly compared with single-dose dog studies. The tablets were orally administered to a group of four dogs and the gallium plasma levels were measured at different time points after administration. The results of the two gallium citrate batches and the better performing batch of gallium nitrate (clinical prototype) are shown in Table 1 below.
[0082] Table 1
[0083]
[0084] The bioavailability of two batches of gallium citrate tablets and one batch of gallium nitrate (clinical prototype) tablets with better performance after oral administration to dogs is graphically depicted in figure 1 in. The figure clearly shows that the oral absorption of gallium from each batch of gallium citrate tablets (Tmax≈30-60min) is significantly faster than that of gallium nitrate tablets (Tmax≈90-180min). In this respect, the oral administration of gallium citrate is similar to the IV administration of gallium nitrate. See Le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com