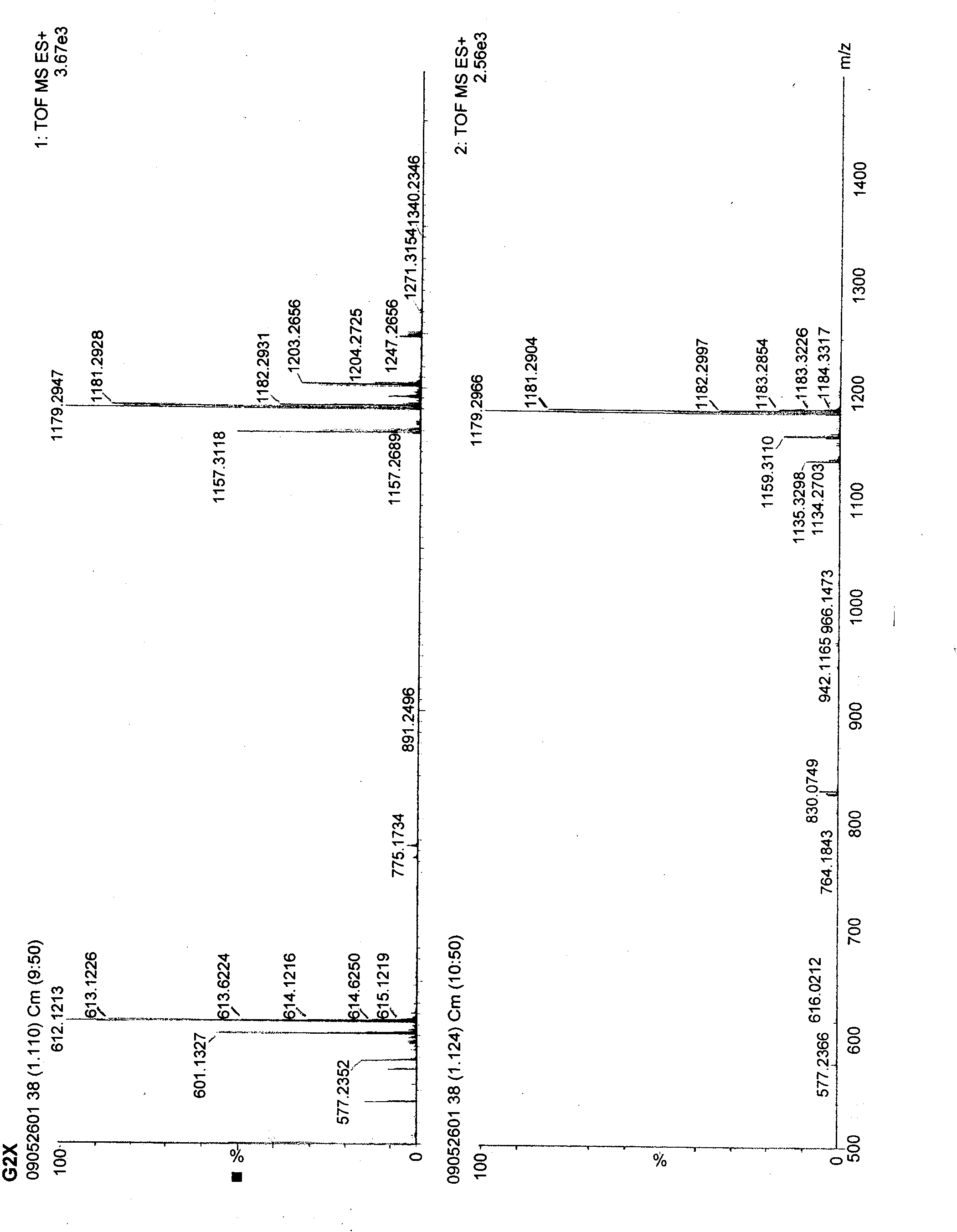
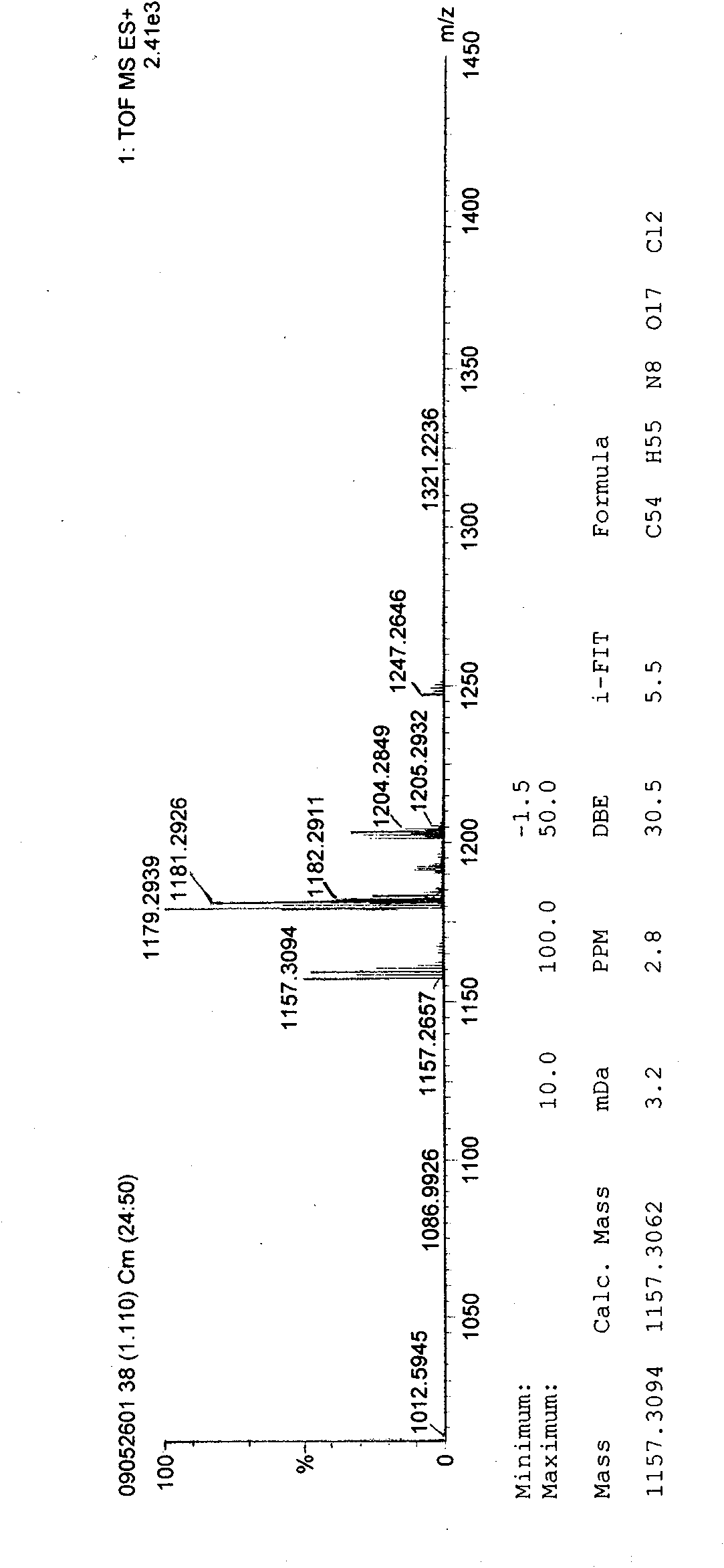
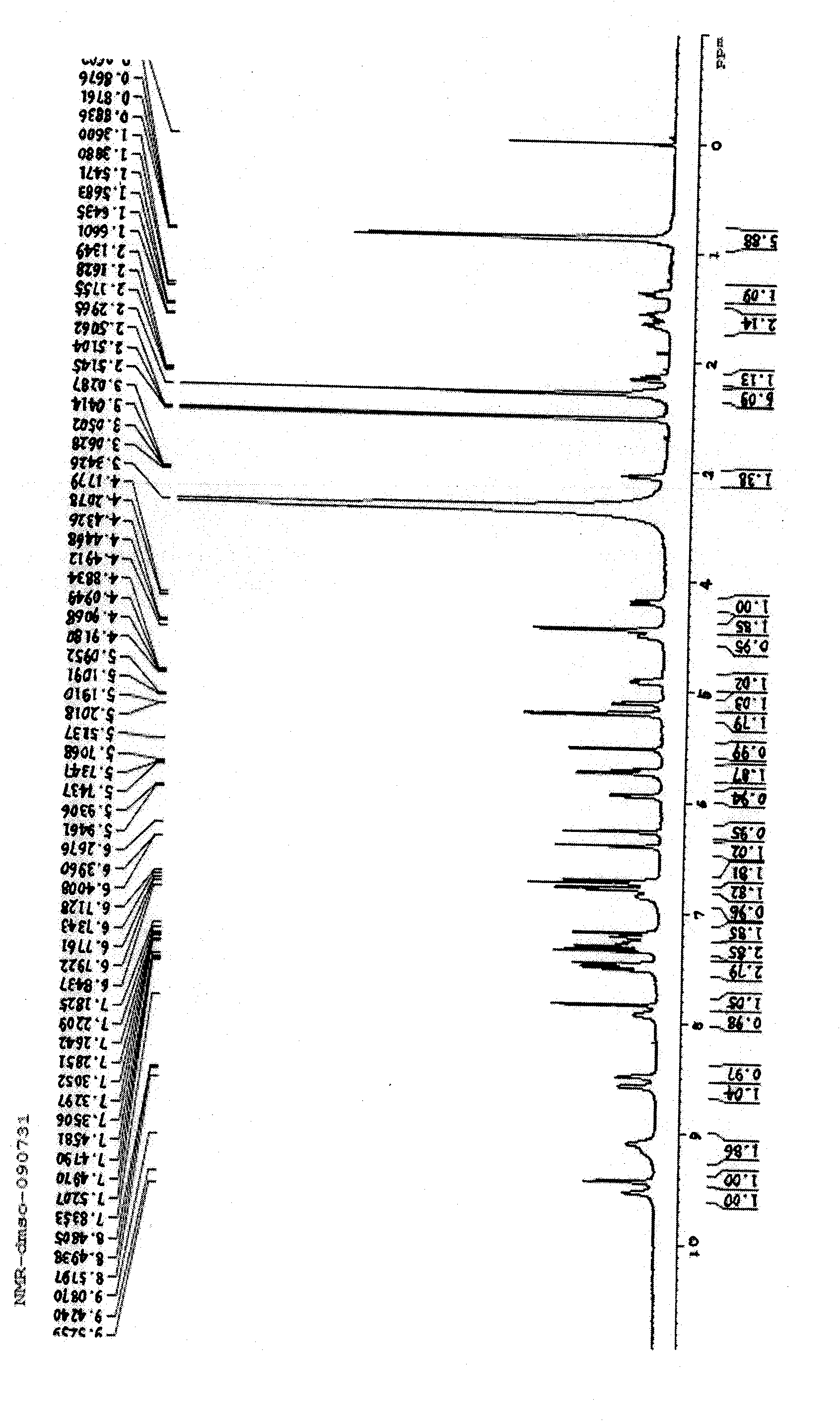
Vancomycin compound
A vancomycin and compound technology, applied in the direction of drug combinations, glycopeptide components, peptides, etc., can solve the problems of inability to prepare oral drugs and affect the application of vancomycin, and achieve high stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 , Inducible expression of N-methyltransferase
[0032] Pick a fresh single clone of the expression strain E.coli BL21(DE3) / pLYLH13, add it to LB medium supplemented with 50 μg / ml kanamycin, and culture overnight at 37°C. Take 1ml of bacterial liquid and add it to 100ml LB containing 50μg / ml kanamycin, cultivate to OD 600 After the temperature is 0.6-0.8, add IPTG with a final concentration of 1 mmol / L, and induce at 30°C for 6 hours. The bacterial cells were collected by centrifugation, and after ultrasonic disruption, the supernatant was collected by centrifugation to obtain a crude enzyme solution.
Embodiment 2
[0033] Example 2 , crude enzyme solution into sugar-free vancomycin
[0034] Using the crude enzyme solution obtained in Example 1, catalyze the reaction of sugar-free vancomycin solution for 24hrs at 25°C, the reaction conditions are as follows:
[0035] The reaction system is (1ml): adenosylmethionine (SAM) 2mmol / L 100μl; sugar-free vancomycin 0.5mg / ml 300μl; crude enzyme solution 100μl, 500μl 50mmol / L Tris / HCl at pH 7.5.
[0036] The solution obtained by the reaction is detected by HPLC, specifically as follows:
[0037] Mobile phase preparation: use 0.2% triethylamine solution and phosphoric acid to adjust the pH to 3.2 as a buffer. Mix the buffer solution with acetonitrile and tetrahydrofuran at a volume ratio of 92:7:1, shake well, and use it as solution A; mix the buffer solution with acetonitrile and tetrahydrofuran at a volume ratio of 39:60:1, shake it well, and use it as solution B. Gas for 20 minutes, set aside. During the elution process, the ratio of liquid ...
Embodiment 3
[0048] Example 3 , Reaction liquid purification
[0049]The reaction solution obtained in Example 2 was centrifuged and the supernatant was collected, and then the centrifuged supernatant was adsorbed using a macroporous adsorption resin Amberlite XAD-1600 column, and gradient eluted with water and ethanol aqueous solutions of different concentrations respectively, and each part was collected. The gradient effluent was detected by HPLC, the target components were combined, and the combined effluent was concentrated under reduced pressure and freeze-dried to obtain a purified crude product.
[0050] Then, 200 mg of the obtained crude product was dissolved in 20 ml of buffer solution C, and then the sample was loaded to the pre-equilibrated C with buffer solution C. 18 In the reverse-phase medium pressure preparative column (flow rate: 5ml / min), then use buffer solution C to elute, and collect the eluent, the effluent is detected by a 254nm ultraviolet absorption detector, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap