Method and pharmaceutical composition for obtaining the plasmatic progesterone levels required for different therapeutic indications
A technology of plasma and plasma concentration, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as progesterone and progesterone not reached
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
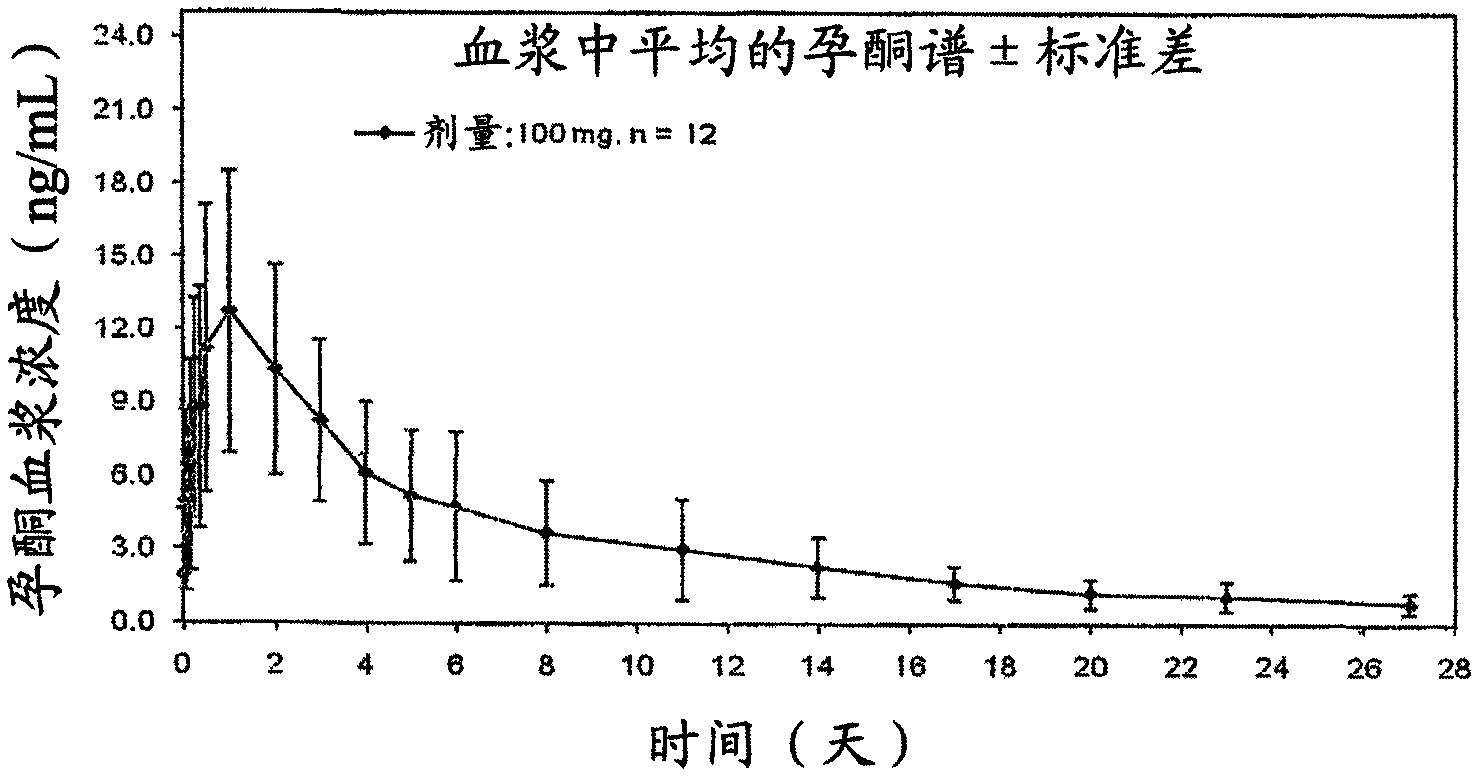
[0034] Single 100 mg injection of progesterone spherical microparticles in injectable suspension.
[0035] A single injection of 100 mg of progesterone spherical microparticles in the form of an injectable aqueous suspension was administered to 12 postmenopausal women. Plasma levels obtained at figure 1 , where it was observed that progesterone plasma concentrations were maintained for up to 7 days at levels appropriate for several treatments requiring said progesterone concentrations.
Embodiment 2
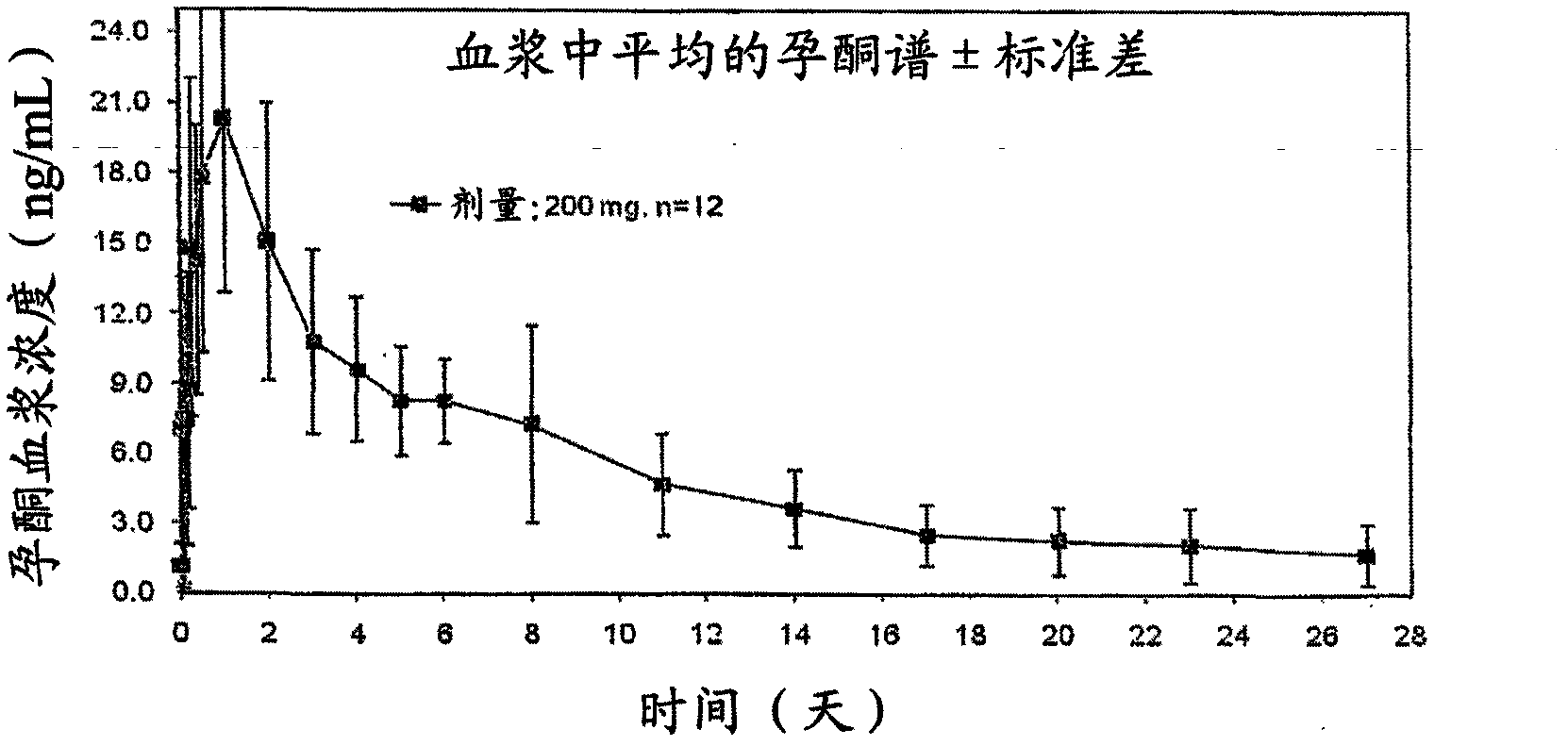
[0037] A single 200 mg injection of progesterone spherical microparticles in injectable suspension.
[0038] A single injection of 200 mg of progesterone spherical microparticles in the form of an injectable aqueous suspension was administered to 12 postmenopausal women. Plasma levels obtained at figure 2 , where it was observed that progesterone plasma concentrations were maintained for up to 7 days at levels appropriate for several treatments requiring said progesterone concentrations and described on page 2.
Embodiment 3
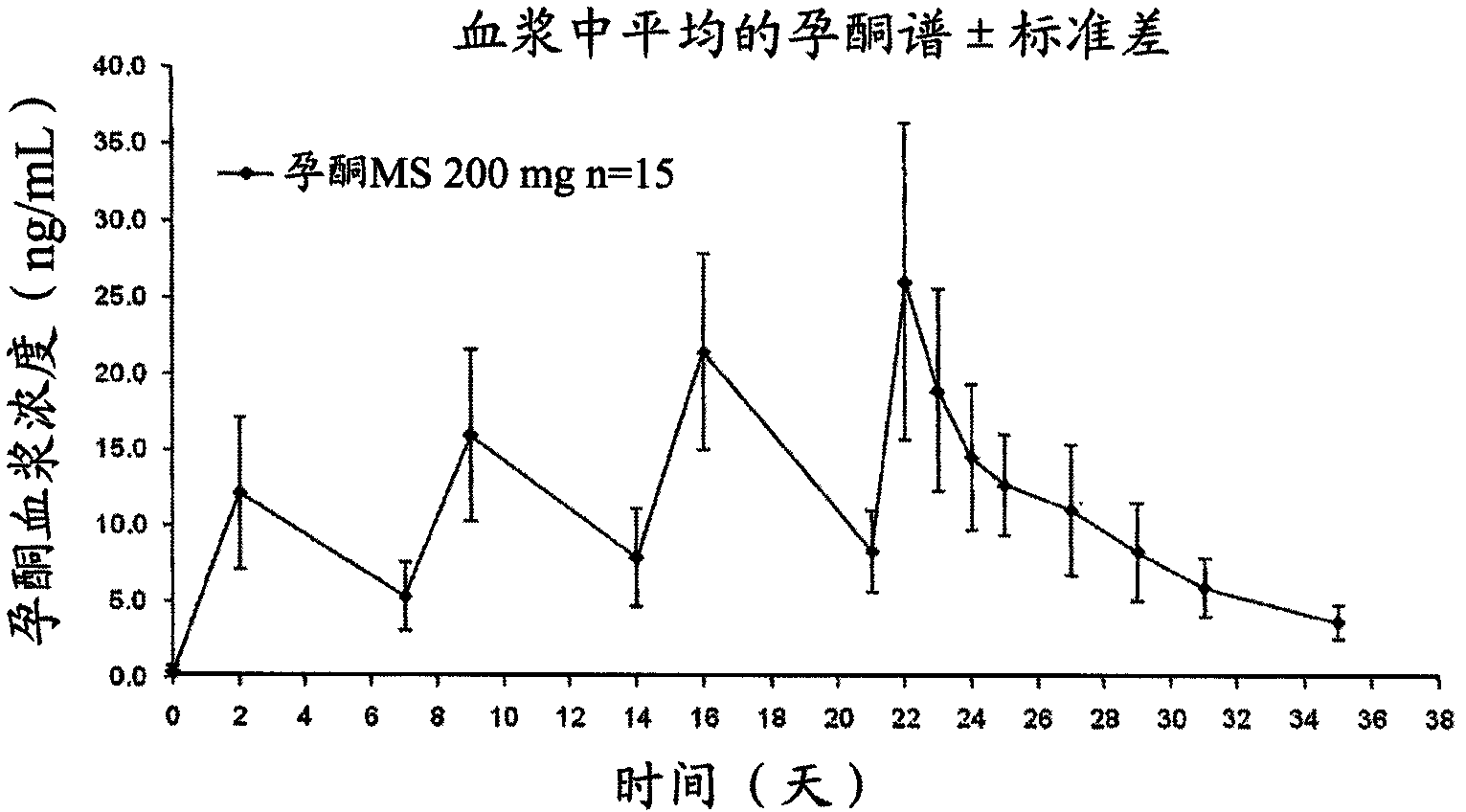
[0040] Repeated 200 mg injections of progesterone spherical microparticles in injectable suspension.
[0041] Four repeated injections of 200 mg of progesterone spherical microparticles were administered as an injectable aqueous suspension to 15 postmenopausal women. Plasma levels obtained at image 3 , where progesterone plasma concentrations were observed to be maintained for up to 7 days at levels appropriate for several treatments requiring said progesterone concentrations and described on page 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com