Antiallergic marine biopolymers
An allergy and composition technology, applied in the fields of physiology and immunology, can solve the problems of large side effects and little effect of treatment of chronic allergic disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Inhibition of TNF-α production by IgE / antigen-stimulated mast cells.
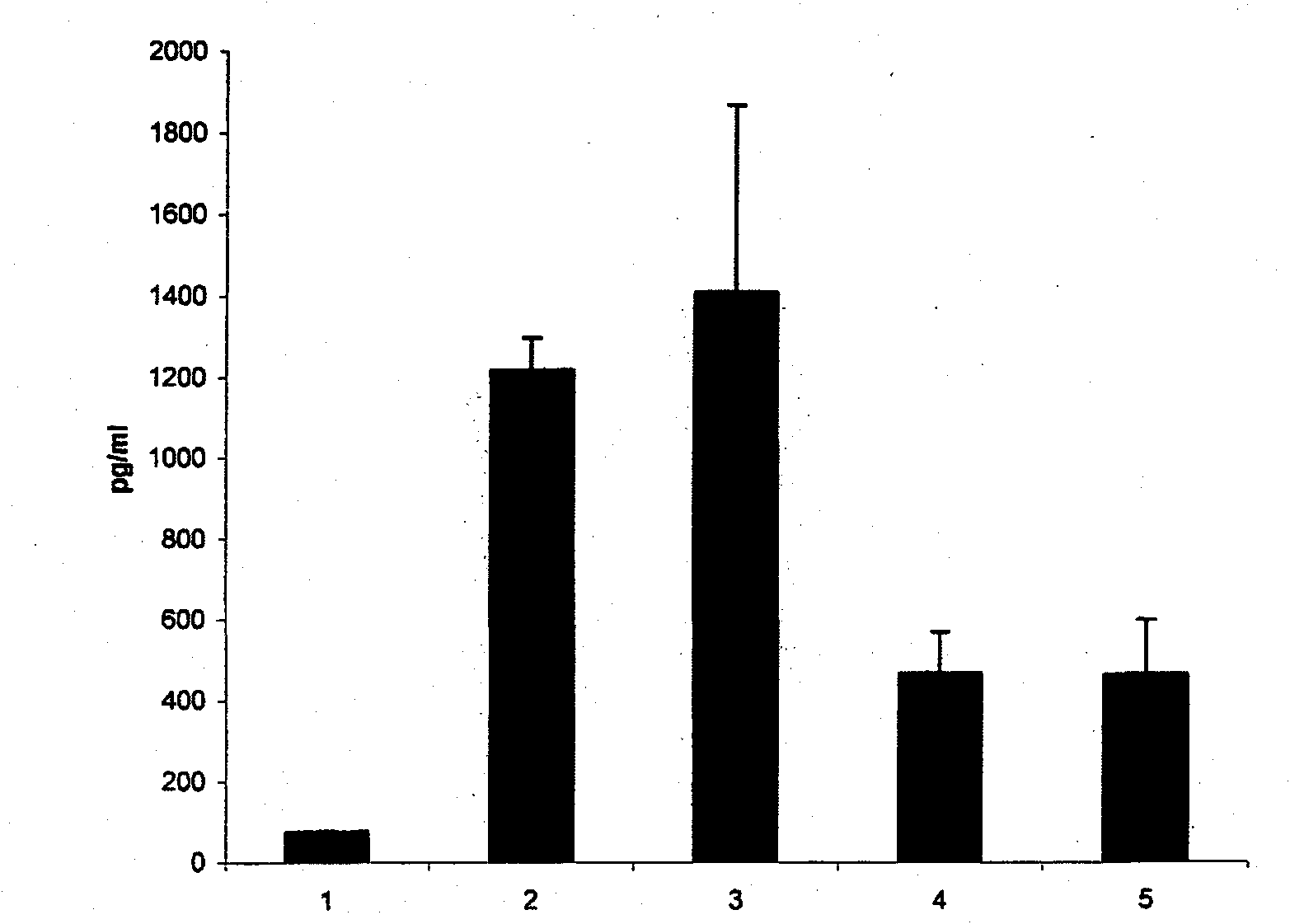
[0024] TNF-[alpha] is a central mediator of the inflammatory response observed in infectious and autoimmune diseases and is released by leukocytes, mast cells, endothelial cells, and several other tissues during injury. Cell-based assays using mouse mast cells stimulated with IgE / antigen complexes showed that both iota-carrageenan and kappa-carrageenan inhibited TNF-α release, whereas lambda-carrageenan did not ( figure 1 ).
[0025] CFTL12 mast cells were incubated with iota-carrageenan or kappa-carrageenan at a concentration of 200 μg / ml. After 60 minutes the cells were stimulated with IgE / antigen complexes. Cells were incubated at 37°C for 6 hours, and TNF-α in the supernatant was determined by a commercial mouse TNF-α ELISA (Bender-Med-Systems). Error bars represent standard deviation among 4 independent wells.
[0026] 1 = no stimulation; 2 = IgE / antigen stimulation; 3 = λ-carrag...
Embodiment 2
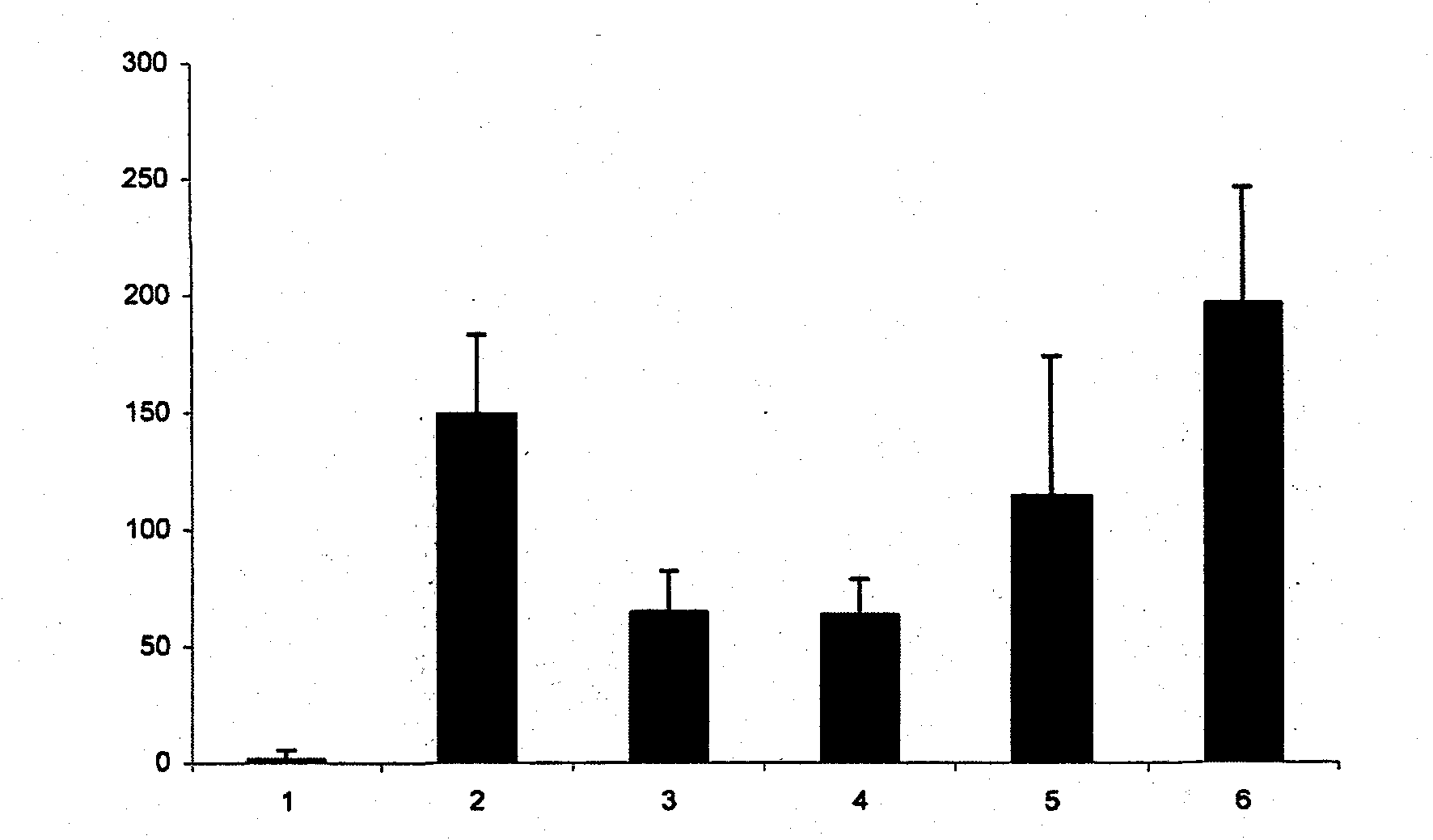
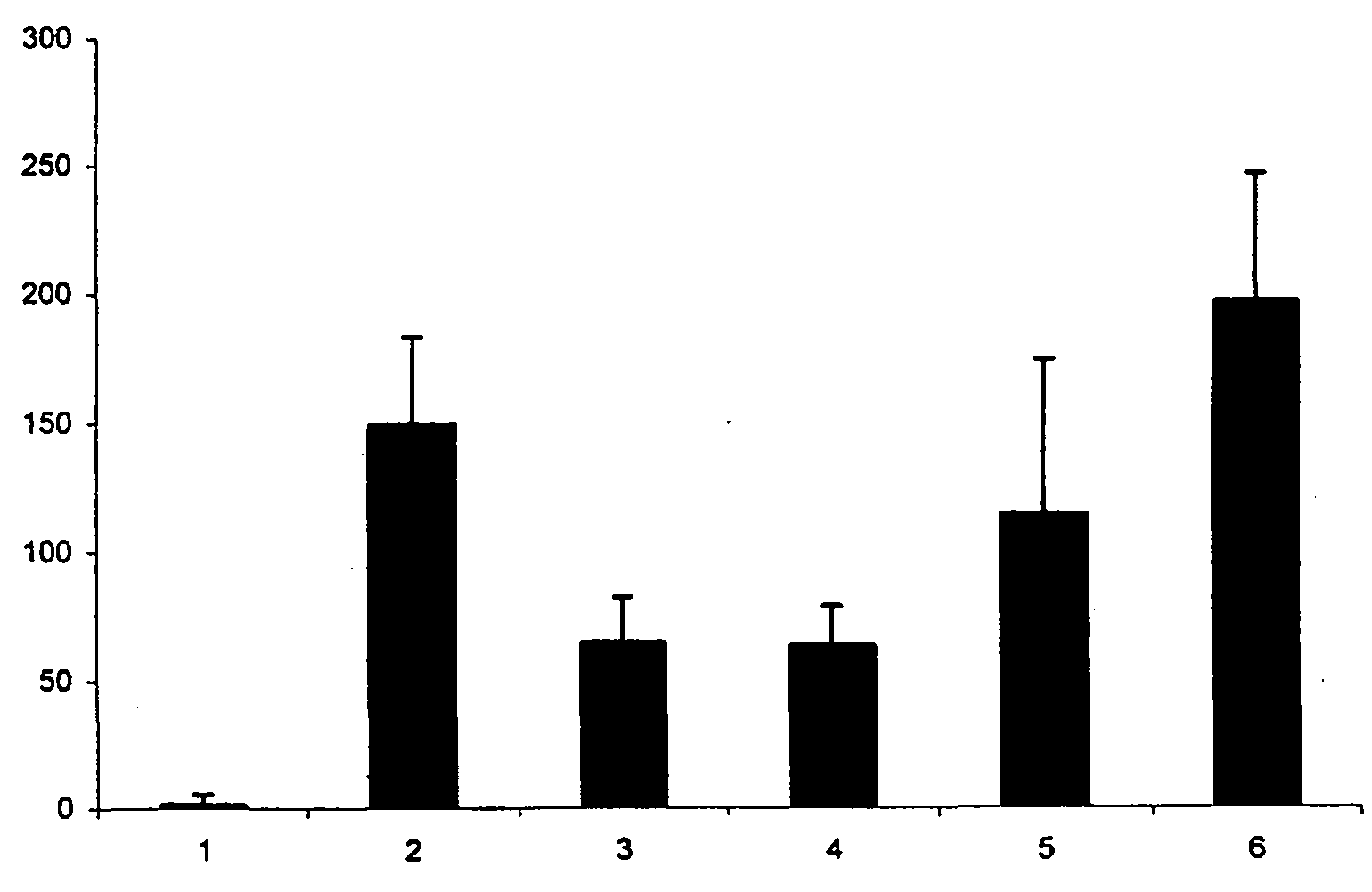
[0027] Example 2: Dose-dependent inhibition of TNF-α production by IgE / antigen-stimulated mast cells
[0028] Mast cells were incubated with various concentrations of iota-carrageenan. After 60 minutes the cells were stimulated with IgE / antigen complexes. Cells were incubated at 37°C for 6 hours, and TNF-α in the supernatant was determined using a commercially available mouse TNF-α ELISA (Bender-Med-Systems). The result is as figure 2 shown. Error bars represent standard deviation among 4 independent wells. 1 = unstimulated; 2 = stimulated with IgE / antigen; 3 = iota-carrageenan at 200 μg / ml; 4 = iota-carrageenan at 66 μg / ml; 5 = iota-carrageenan at 6.6 μg / ml Vegetarian gum; 6 = iota-carrageenan at 0.6 μg / ml. The Y-axis represents the concentration of TNF-α in pg / ml.
Embodiment 3
[0029] Example 3: Application of Carrageenan Nasal Spray in Improving Allergy Symptoms
[0030] A 29-year-old patient with a well-documented history of type I anaphylaxis, intense symptoms of allergic rhinitis and hypersensitivity to several plant pollens led him to the following regimen: nightly administration of iota-carrageenan nasal spray, and increase the dose during pollen season. The patient reported that the frequency of sneezing had decreased significantly during the use of the nasal spray and that he could sleep undisturbed again. In addition, the patient reported a decrease in inflammation of the nasal mucosa. The patient further reported no need for additional medications, particularly intranasal decongestants, antihistamines, and corticosteroids, in stark contrast to previous pollen seasons.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com