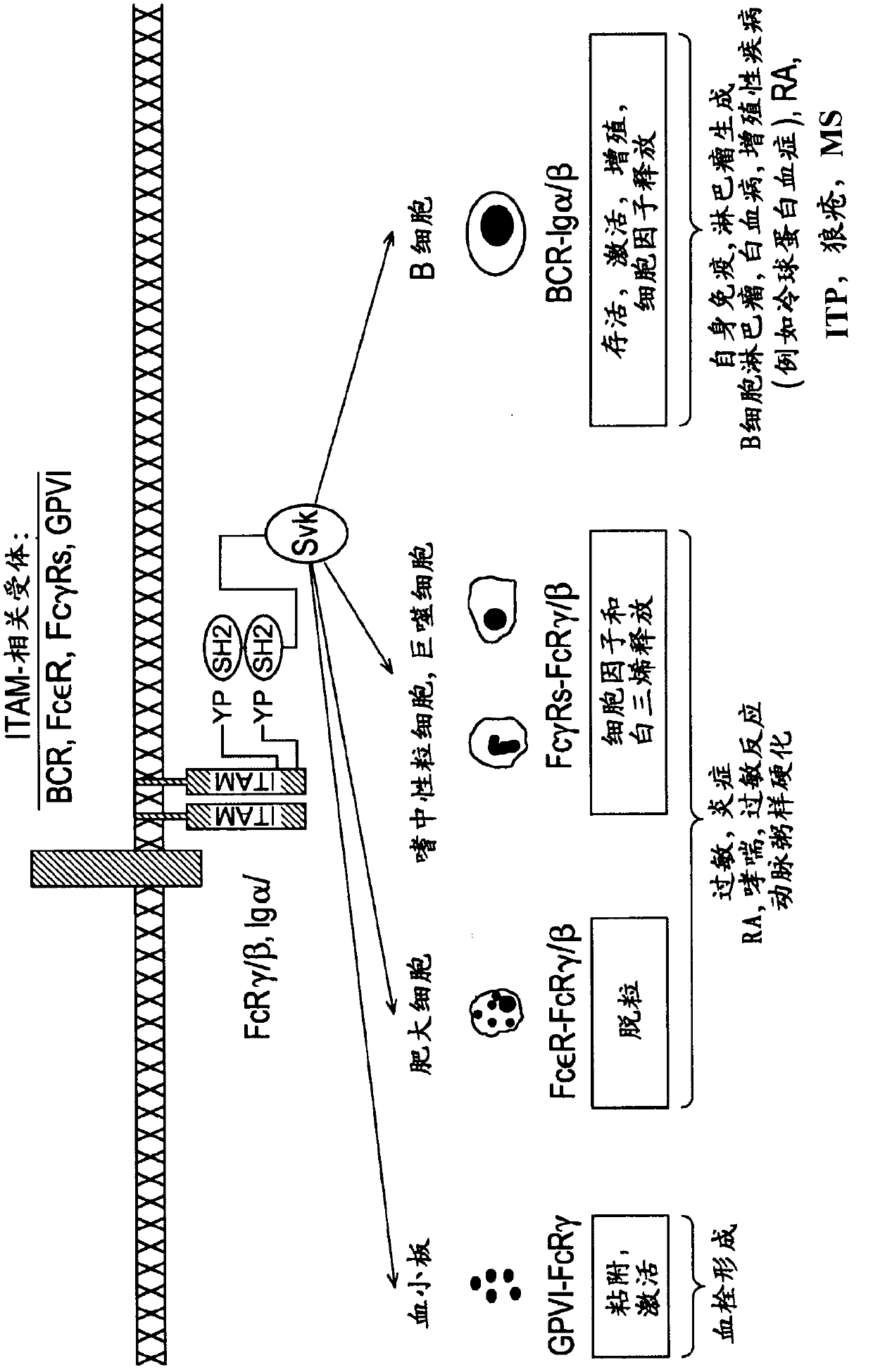
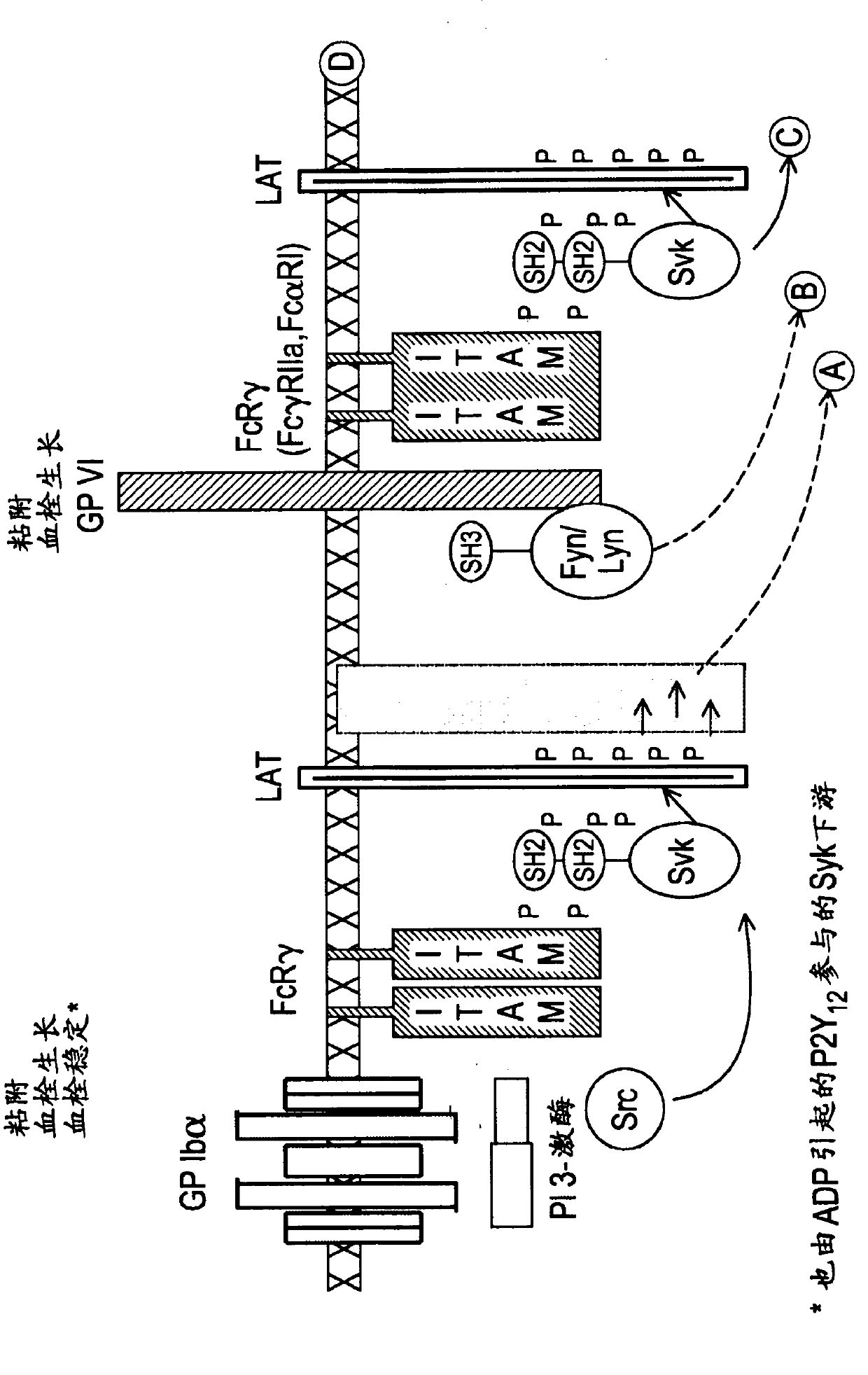
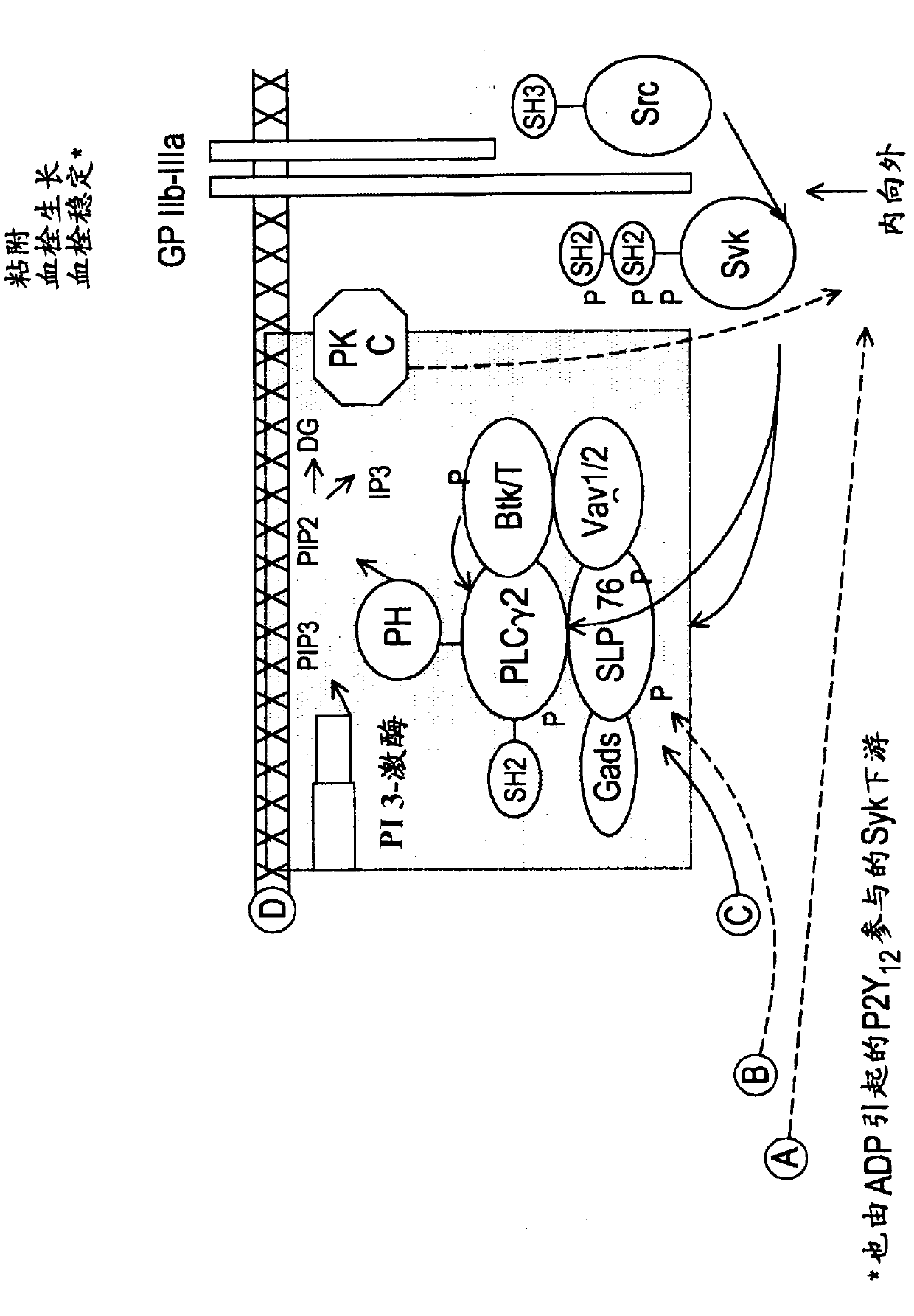
Inhibitors of protein kinases
A compound, alkyl technology, applied in the field of treating diseases characterized by unwanted thrombosis, preparing the compounds described in this article, can solve the problems of Src kinase inactivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0335] a. Compound
[0336]In one group of embodiments, the present invention provides a compound having formula (I), a tautomer or a pharmaceutically acceptable salt thereof:
[0337]
[0338] in:
[0339] Y 1a selected from N, CH and C;
[0340] Z 1a selected from the bond, -N(C 1-4 Alkyl)-, -SO 2 -, -CO-, -NR 4d SO 2 -, heterocyclyl, heterocyclic carbonyl and heterocyclic sulfonyl;
[0341] R 1a selected from:
[0342] (a) hydrogen;
[0343] (b)C 1-8 Alkyl, optionally 1-3 selected from amino, hydroxyl, C 1-8 Alkoxy, heterocyclyl, aminocarbonyl, amino C 1-8 Substitution of alkoxy, aryl and heteroaryl groups;
[0344] (c)C 3-8 Cycloalkyl, optionally substituted by 1-3 amino substituents;
[0345] (d) aryl, optionally selected from 1-3 C 1-8 Alkyl, C 1-8 Alkoxy, C 1-8 Alkylamino, C 1-8 Alkylcarbonylamino, aminocarbonyl C 1-8 Alkoxy, aminosulfonyl, aminocarbonyl, amino C 1-8 Alkylenecarbonyl C 1-8 Alkoxy and halogen substituent substitution;
[0346] ...
Embodiment 1
[0802] N2-(1H-indazol-6-yl)-N4-methyl-5-(pyridin-4-yl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamine
[0803]
[0804] To a suspension of 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (2.0 g, 10.6 mmol) in DCM (32 mL) was added NIS (2.4 g, 10.6 mmol) at room temperature . After stirring for 1 hour, the obtained precipitate was collected by filtration to obtain 2,4-dichloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (1.8 g).
[0805] To a mixture of 2,4-dichloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (1.80 g, 5.73 mmol) in DCM (20 mL) was added TsCl (1.09 g, 5.73 mmol) and TEA (1.60 mL, 11.46 mmol), followed by DMAP (70 mg, 0.573 mmol). After stirring at room temperature for 1 h, the solution was concentrated and the residue was dissolved in EtOAc and H 2 Partition between O, separate the organic phase with 1N HCl, 5% NaHCO 3 Washing, drying and concentration gave 2,4-dichloro-5-iodo-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine (2.0 g).
[0806]To a mixture of 2,4-dichloro-5-iodo-7-tosyl-7H-pyrrolo[2,...
Embodiment 2
[0811] 1-(4-(4-(4-(methylamino)-5-(pyridin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-2-ylamino) phenyl)piperazin-1-yl)ethanone
[0812]
[0813] To 2-chloro-N-methyl-5-(pyridin-4-yl)-7-tosyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (0.044g, 0.11mmol) To a mixture of nBuOH (0.6 mL) was added 1-(4-4-aminophenyl)piperazin-1-yl)ethanone (0.047 g, 0.22 mmol) and TMSCl (0.043 mL, 0.33 mmol). After heating at 115 °C for 48 h, the mixture was purified by preparative HPLC to obtain 1-(4-(4-(4-(methylamino-5-(pyridin-4-yl)-7H-pyrrolo[2,3- d] pyrimidin-2-ylamino)phenyl)piperazin-1-yl)ethanone (0.015 g), MS (MH 443.3), λ = 259.4, 302.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com