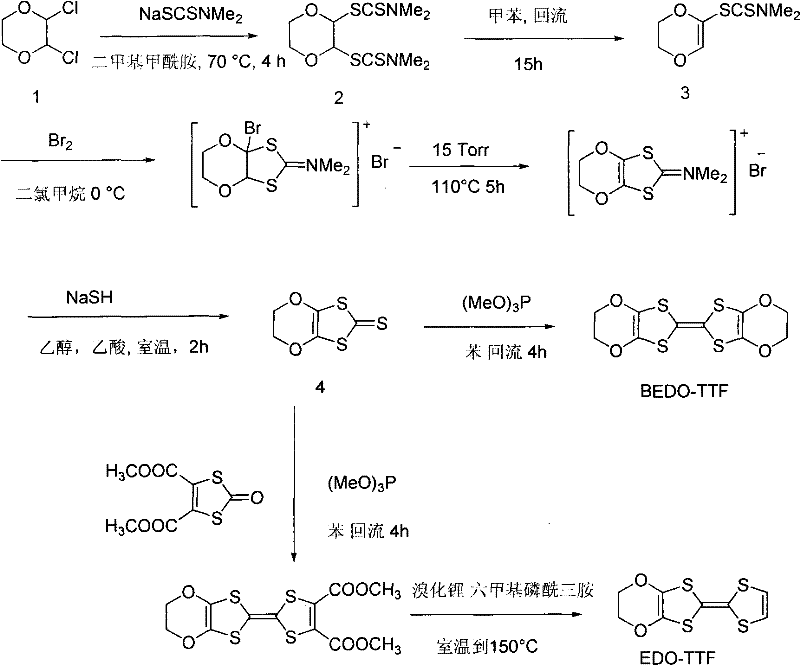
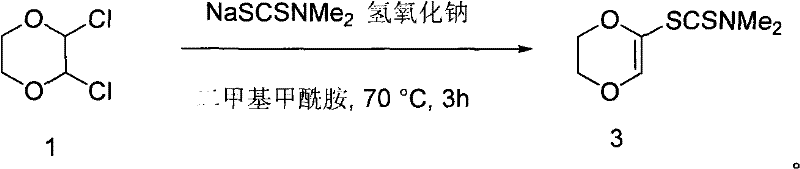
Method for preparing compound 2-N, N-dimethylaminodithioformic acid-2, 3-dihydro-1, 4-dioxane
A technology of dimethyl dithiocarbamate and sodium dimethyl dithiocarbamate, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 2,3-dichloro-1,4-dioxane (40.65 g, 0.26 mol), NaSCSN (CH 3 ) 2 .2H 2 O (49.8 grams, 0.35 moles) and sodium hydroxide (5 grams, 0.125 moles) in DMF (200 milliliters) were slowly heated to 70 DEG C in a there-necked flask, and stirred at this temperature for 3 hours, a large amount of solids were separated out, The resulting solid was filtered, then the solid was dissolved in chloroform, washed three times with saturated brine, then washed twice with water, dried over anhydrous magnesium sulfate, and the chloroform was removed to obtain 2-N,N-dimethyl in the form of colorless crystals. Dithiocarbamate-2,3-dihydro-1,4-dioxane (47 g, 77% yield).
[0017] The melting point is 170-171°C;
[0018] 1 H-NMR (CDCl 3 , 400Hz) 6.47 (s, 1H), 4.28-4.30 (m, 2H), 4.19-4.21 (m, 2H), 3.53 (s, 3H), 3.39 (s, 3H).
[0019] 13 C-NMR 195.2 137.5, 128.2 64.8, 84.2, 45.5, 41.7.
Embodiment 2
[0021] 2,3-dichloro-1,4-dioxane (40.65 g, 0.26 mol), NaSCSN (CH 3 ) 2 .2H 2 O (49.8 grams, 0.35 moles) and sodium hydroxide (1 gram, 0.025 moles) were slowly heated to 70° C. in a three-necked flask in DMF (200 milliliters), and stirred at this temperature for 3 hours, a large amount of solids were separated out, The resulting solid was filtered, then the solid was dissolved in chloroform, washed three times with saturated brine, then washed twice with water, dried over anhydrous magnesium sulfate, and the chloroform was removed to obtain 2-N,N-dimethyl in the form of colorless crystals. Dithiocarbamate-2,3-dihydro-1,4-dioxane (47 g, 77% yield).
[0022] The melting point is 170-171°C;
[0023] 1 H-NMR (CDCl 3 , 400Hz) 6.47 (s, 1H), 4.28-4.30 (m, 2H), 4.19-4.21 (m, 2H), 3.53 (s, 3H), 3.39 (s, 3H).
[0024] 13 C-NMR 195.2 137.5, 128.2 64.8, 84.2, 45.5, 41.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com