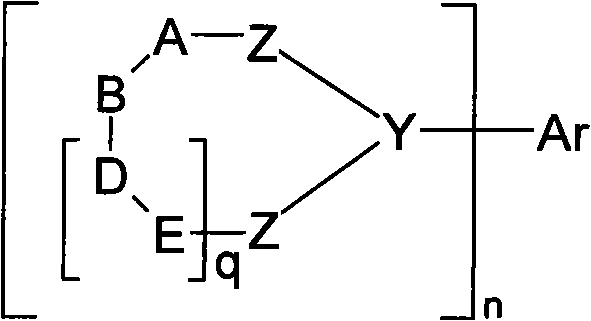
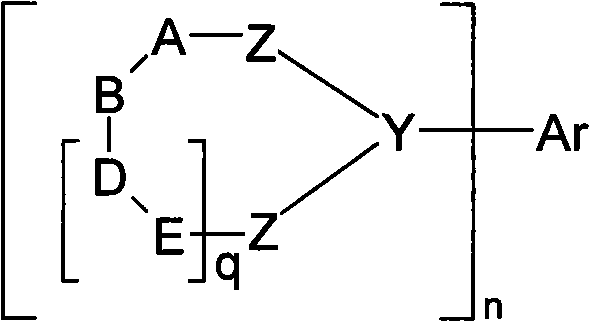
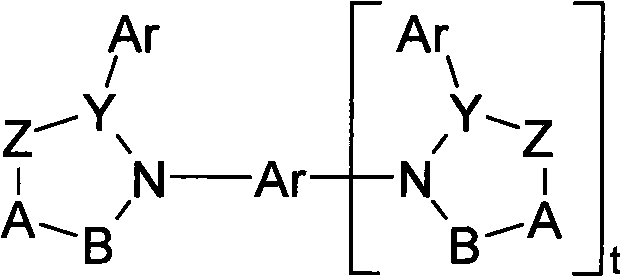
Organic electroluminescent devices
An electronic device and general formula technology, applied in the field of organic electroluminescence devices, can solve the problem of not satisfying the disclosure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0178] Example 1a: Synthesis of 1,4-bis(phosphonochloride)benzene:
[0179]
[0180] First 55.2 g (232 mmol) of 1,4-bis(phosphonic acid)benzene were added to 1400 ml of dichloromethane, followed by 10 drops of DMF. A solution of 86.3 ml (1020 mmol) of oxalyl chloride in 400 ml of dichloromethane was added dropwise at room temperature, and the mixture was stirred at 45° C. for 5 h. The solvent was removed under vacuum and the product was recrystallized from hexane under protective gas. Yield: 70 g (227 mmol), 98%.
[0181] The following compounds can be obtained similarly:
[0182]
Embodiment 2
[0183] Example 2: General Synthesis of N, N'-Diaryl-1,2-Phenylenediamine
[0184] 1.06g (4.75mmol) of Pd(OAC) 2 and 14.46 ml (14.46 mmol) of tri-tert-butylphosphine (1M solution in toluene) were added to 660 ml of degassed toluene, and the mixture was stirred for 5 minutes. Then 240 mmol of 1,2-dibromobenzene derivative, 505 mmol of arylamine and 67.22 g (700 mmol) of sodium tert-butoxide were added to the solution, which was then degassed and stirred again at 140 °C in a protective atmosphere 10h. After the solution was cooled, 600 ml of ammonium chloride solution and 150 ml of ethyl acetate were added, the phases were separated, washed with water, and washed with MgSO 4 Dry and evaporate. The solid was dissolved in toluene and filtered through celite. The crude product was stirred and washed with hot n-heptane.
Embodiment 3
[0185] Example 3: Synthesis of 4,5-dimethyl-N, N'-diphenyl-1,2-phenylenediamine
[0186]
[0187] According to Example 2, from 63.3 g (240 mmol) of 1,2-dibromo-4,5-xylene and 46 ml (505 mmol) of aniline, the synthesis was carried out according to the general procedure. The precipitated solid was recrystallized from toluene / acetonitrile (5:1), and the residue was washed with methanol to obtain 65 g (223 mmol) of a crystalline solid. The overall yield is 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap