Formulations for treating eye disorders
A technology for mycophenolate sodium and ocular diseases, applied in the field of ocular solutions, can solve the problems of low bioavailability and intolerance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0065] Example 1: Preparation of NaMPA ophthalmic solution.
[0066] Ophthalmic Solution 1.4% MPA Ophthalmic Solution, Sodium Salt
[0067]
[0068] Preparation procedure. About 80% of the final volume of pure water was heated to about 80°C. Add glycerol and mycophenolic acid to water and mix to disperse. The heat was turned off and 10% NaOH was immediately added to the batch and mixed until the MPA was dissolved. Alternatively, pure water (approximately 80% final volume), 10% NaOH and glycerol were mixed and heated to approximately 80°C. The heat was turned off, then mycophenolic acid was added and mixed to dissolve. Pure water was added to about 95% of the batch volume, and the composition was mixed while cooling to room temperature. The pH was measured with a calibrated pH meter and adjusted with NaOH / HCl if necessary. Pure water was added to 100% of the batch volume and osmolarity was measured. Appropriate filters are used for clarification and sterilization.
...
example 2
[0076] Example 2: Ophthalmic Formulations Containing EDTA
[0077] The table below provides various NaMPA ophthalmic formulations with additive EDTA and various levels of NaCl.
[0078] Table 1: Ophthalmic Solutions Containing EDTA
[0079]
[0080]
[0081] The table above shows the ophthalmic NaMPA formulations used in the animal studies including the efficacy studies described below. The first three ingredients (NaMPA, NaCl, and disodium edetate (EDTA)) are shown as % final concentration in weight per volume (w / v). The formulations were adjusted to the desired pH using NaOH or HCl as indicated, and to a final volume of 100 ml with purified water. Other volumes of ophthalmic NaMPA formulations can be prepared using the formulation outlined in Table 1. Under "NaMPA", the final concentration of MPA is shown in parentheses. For example, Formulation 3 refers to the 1% NaMPA formulation. The 1% NaMPA formulation contains 1% final concentration of MPA (active ingredient...
example 3
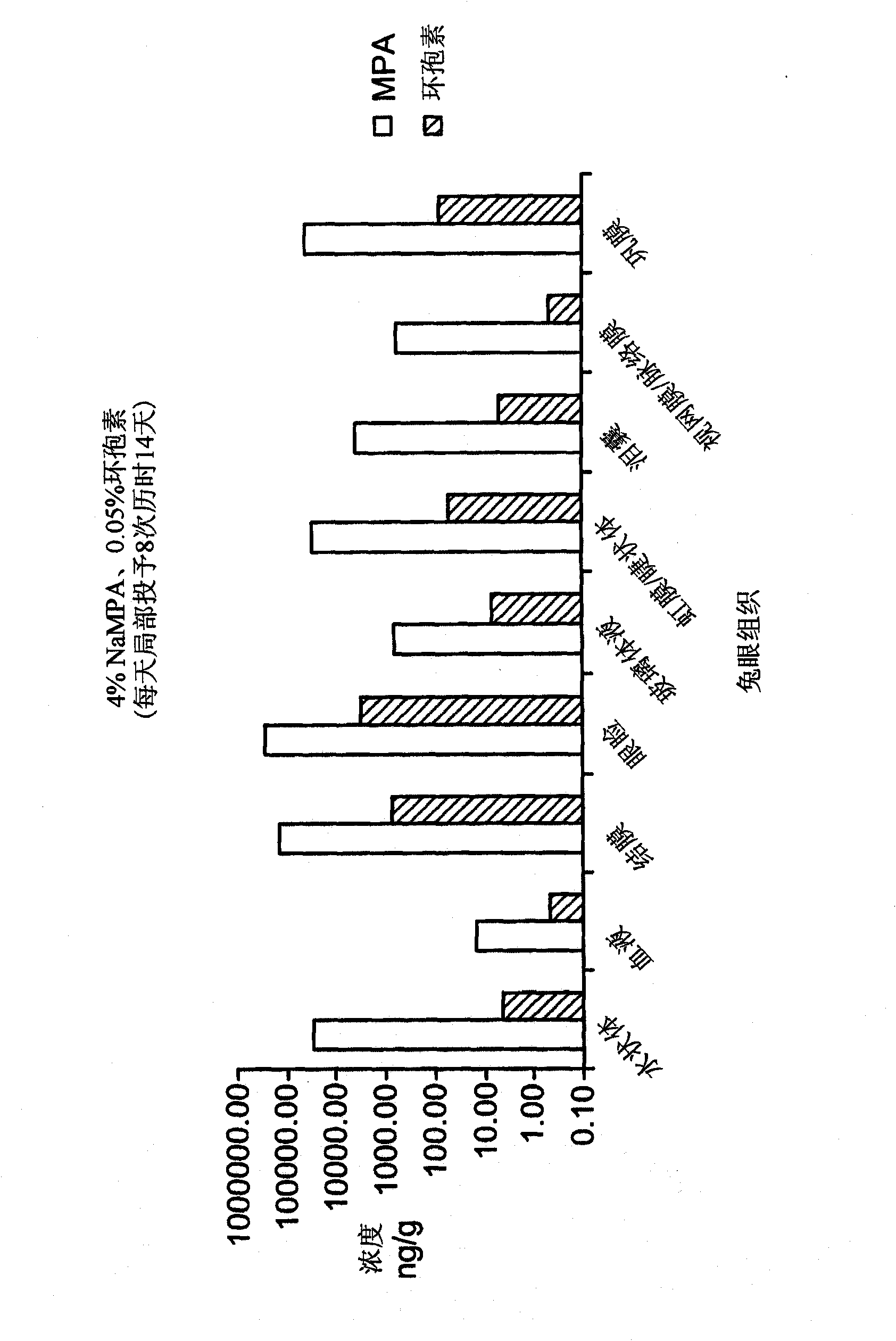
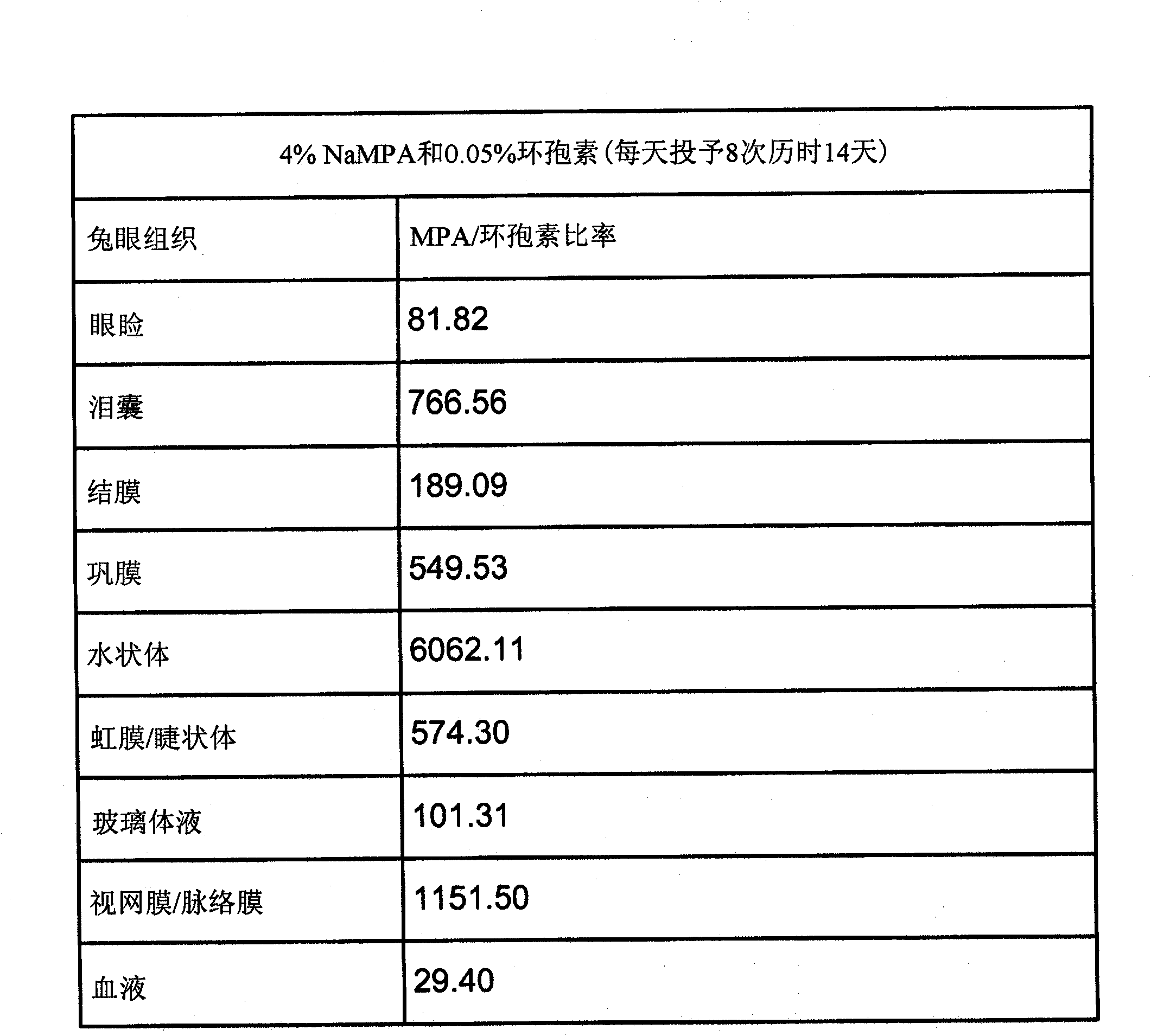
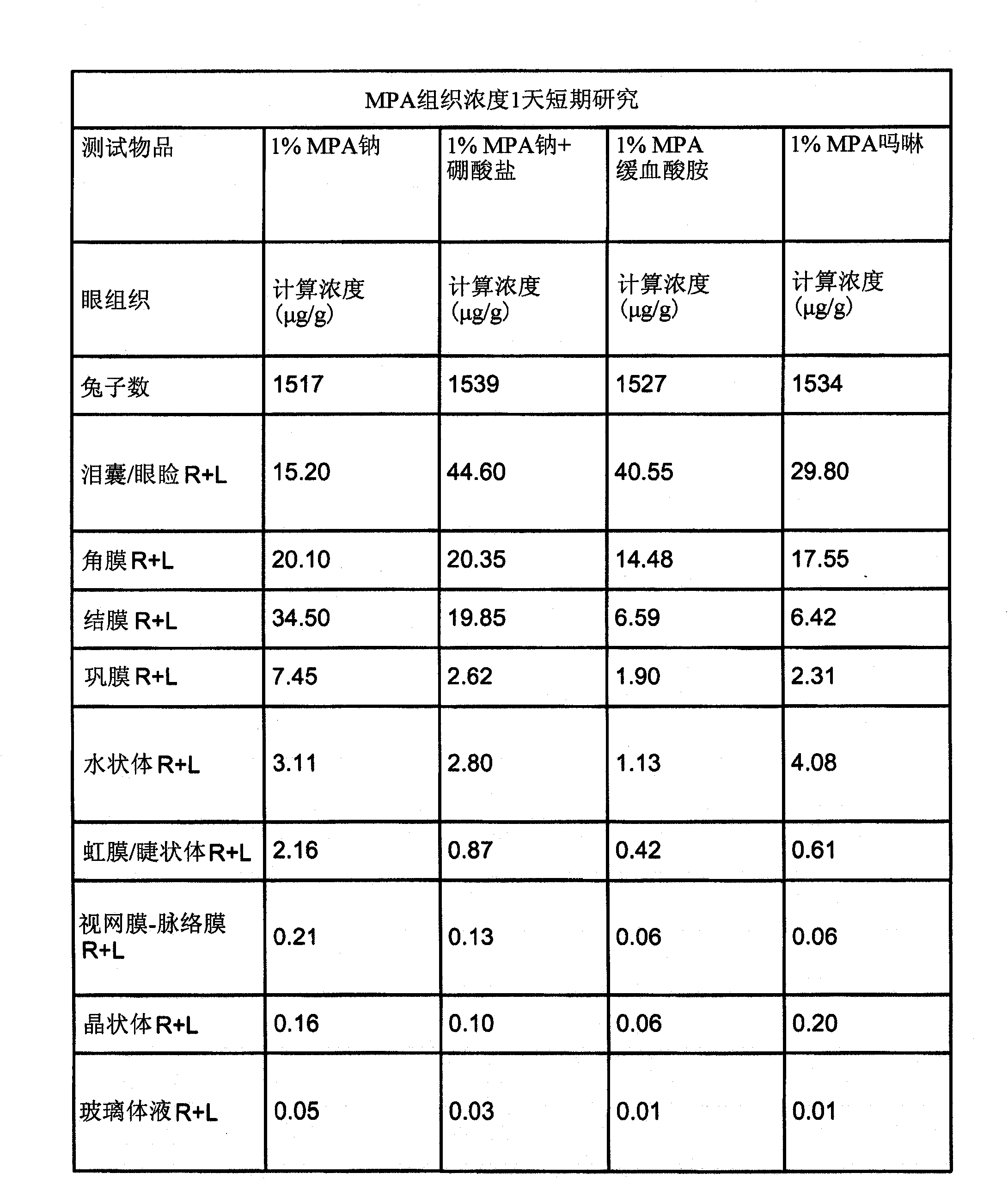
[0083] Example 3: Study of Tolerability and Ocular Tissue Penetration of MPA-Containing Formulations (Compared to Cyclosporine).
[0084] The purpose of this study was to evaluate the ocular tolerance and ocular tissue penetration of eight MPA formulations following one-day topical instillation eight times a day in the eyes of New Zealand White rabbits. Eight test article formulations, a negative control article (2.4% glycerol), and a positive control article are provided in Table 2 Manufactured by Allergan Inc., Irvine, CA, US (Allergan). The study was conducted in two phases, using four test articles and two control articles in each phase. Naive animals were used in both phases. Animals were placed in treatment groups as indicated in Tables 2, 3 and 4.
[0085] Table 2: Treatment Group Assignment: Phase 1
[0086]
[0087]
[0088] MPA = mycophenolic acid.
[0089] Table 3: Treatment Group Assignment: Phase 2
[0090]
[0091] MPA = mycophenolic acid.
[009...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com