Monoclonal antibodies to fibroblast growth factor receptor 2
A technology of monoclonal antibody and antibody, which is applied in the fields of antibody, antitumor drug, biomaterial analysis, etc., and can solve the problem of anti-tumor activity of anti-FGFR2 antibody that has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Reagents and Assays
[0071] Preparation of Flag-FGF1, FLAG-FGF2 and FLAG-FGF7 . Synthesis of human FGF1 (in a 155 amino acid form; Chiu et al., Oncogene 5:755-1990) and human FGF2 (in a 155 amino acid form; Sommer et al., Biochem.Biophys.Res.Comm. 144:543, 1987 ) DNA sequence (GenScript, Inc), then amplified by PCR to have an N-terminal Flag peptide tag, and cloned into a derivative of the pET vector (Invitrogen) using standard molecular biology techniques. These plasmids were transformed into E. coli BL21(DE3) cells, and the expression of FGF1 or FGF2 was induced using 1 mMIPTG. The level of FGF expression was determined using FGF1 or FGF2 specific ELISA kits (R&D Systems). FGF was purified using heparin-sepharose CL-6B beads (Amersham Biosciences) as described (Wiedlocha et al., Mol. Cell. Biol., 16:270, 1996). Similarly, the gene for human FGF7 (the precursor form of 194 amino acids; Finch, P.W. et al., Science 245:752, 1989) was synthesized and PCR-...
Embodiment 2
[0075] Example 2: Production of monoclonal antibodies to FGFR2
[0076] By injecting 20 or 22 injections of FGFR2(beta)IIIb-Fc (initial dose 10 g / footpad, then 5 μg / footpad) at mouse hind footpads at 1-week intervals, or with 17 doses of FGFR2IIIc-Fc (initial Dose 10 μg / footpad, then 5 doses of 2 μg, then 5 μg), followed by 5 doses of FGFR2(β)IIIc-Fc (5 μg / footpad) to immunize Balb / c mice (5-6 week old female), antigen suspension In MPL / TDM (Sigma-Aldrich). Three days after the last injection, popliteal lymphoid cells were extracted and fused with P3 / X63-Ag8U1 mouse myeloma cells at a ratio of 1:1 using the Hybrimune Electrofusion System (Cyto PulseSciences). Hybridomas were selected by adding 2x HAT (Sigma) after 24 hrs. Ten days after fusion, hybridoma culture supernatants were screened for the ability to bind FGFR2IIIb-Fc but not human IgG using ELISA. Selected mAbs were then screened for their ability to recognize FGFR2IIIb on the human gastric tumor cell line SNU-16 ...
Embodiment 3
[0078] Example 3: Properties of anti-FGFR2 mAbs
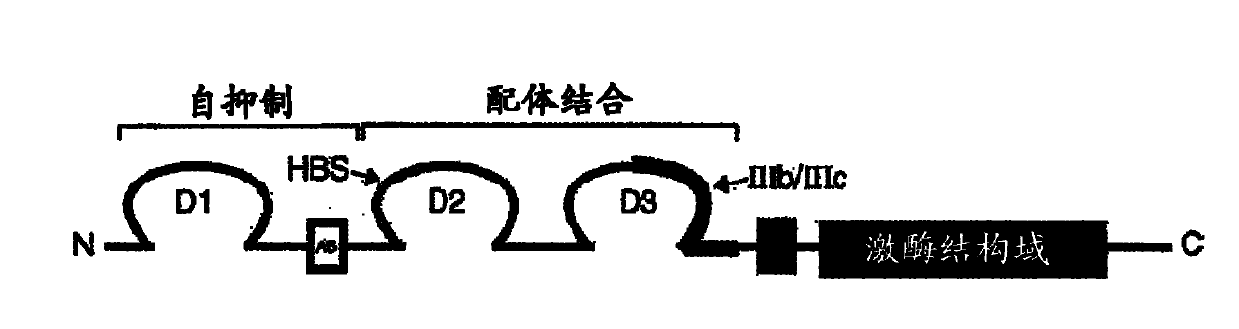
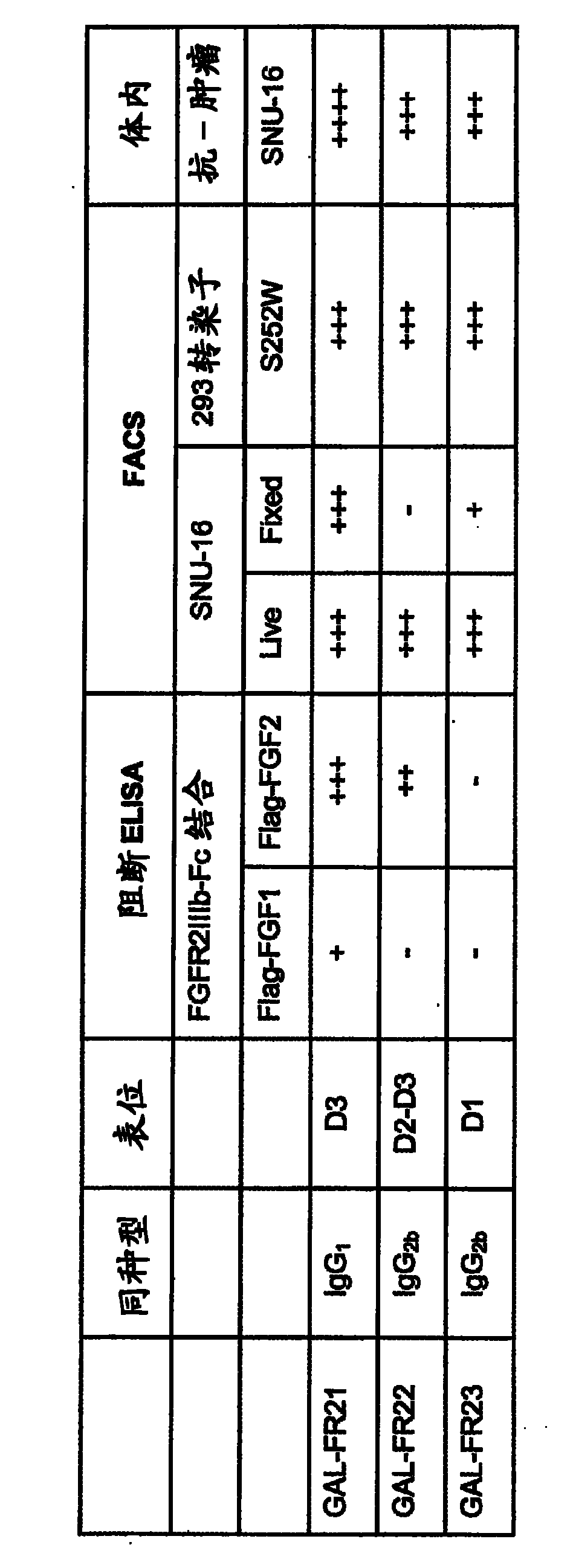
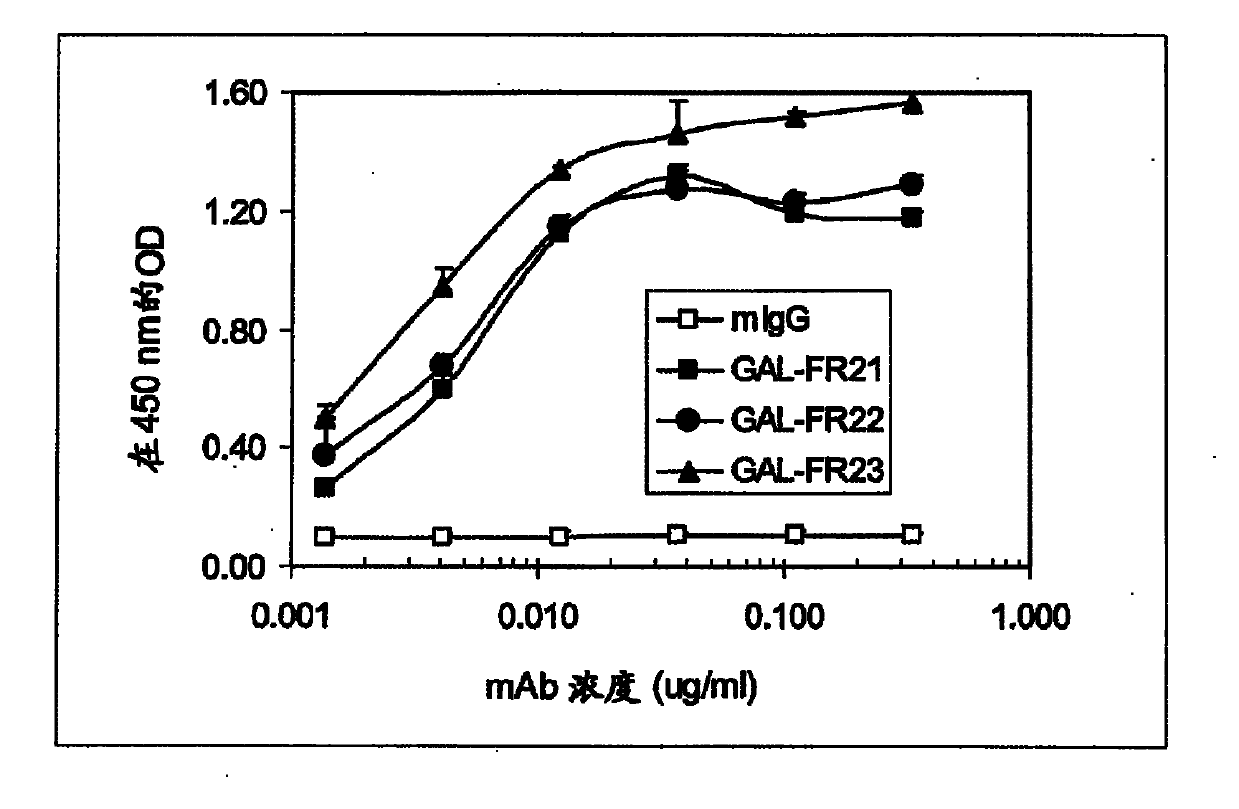
[0079] like image 3 As can be seen in the ELISA assay described in Example 1, all three selected mAbs GAL-FR21, GAL-FR22 and GAL-FR23 bound well to FGFR2IIIb. By using four different forms of FGFR2-Fc in ELISA, it was determined that each of these mAbs has a different binding mode and thus a different epitope ( Figure 4 ). GAL-FR21 binds the alpha and beta forms of FGFR2IIIb (ie, with and without D1), but not FGFRIIIc. Therefore, the epitope cannot possibly involve D1, and must involve D3IIIb, and thus may be encompassed in either D3IIIb or D3. GAL-FR22 also binds the α and β forms, but in IIIb and IIIc contexts, therefore, the putative epitope is covered in D2-D3IIIa, or definitely in D2-D3. Finally, GAL-FR23 does not bind any β-form, so its epitope must be fully or partially in D1. Thus, mAbs having epitopes in D1 or in D2-D3 or in D3 are encompassed in the present invention.
[0080] To verify that mAbs GAL-FR21, G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com