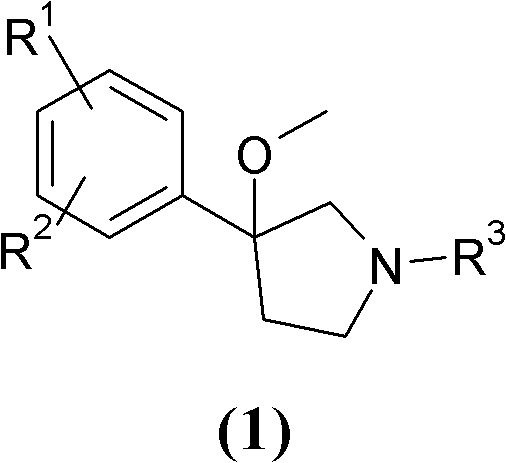
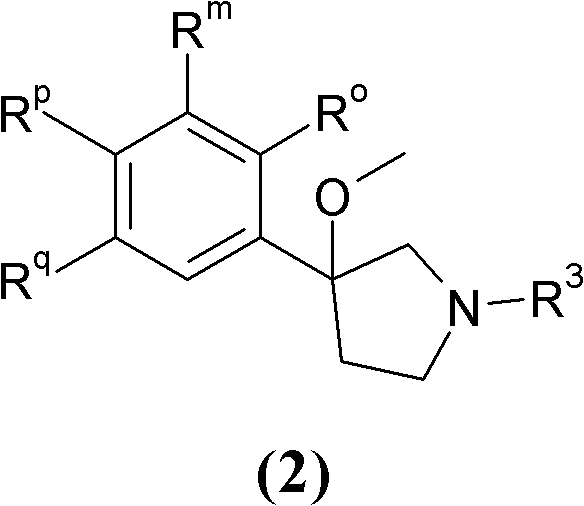
3-phenyl-3-methoxypyrrolidine derivatives as modulators of cortical catecholaminergic neurotransmission
A technology of methoxypyrrolidine and methoxy, which is applied in the field of therapeutic application and treatment of disorders of the central nervous system, and can solve the problems of undisclosed medicinal uses of compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] (+)-3-(3-Chloro-2-fluorophenyl)-3-methoxypyrrolidine
[0157] Add 1-chloroethyl chloromethyl ester (1.42ml, 13.08mmol) to (+)-1-benzyl-3-(3-chloro-2-fluorophenyl)-3-methoxypyrrolidine (1.04 g, 3.25 mmol) in a solution of 1,2-dichloroethane (10 ml), the mixture was heated to reflux for 2 hours, after which the solvent was evaporated. The mixture was dissolved in methanol (20ml), heated to reflux for 1 hour, the solvent was evaporated, and the mixture was subjected to HPLC in waters OBD C18, 5μm (MeOH / 33mM NH 3 , 20:80 to 50:50) to obtain 0.47 g (63%) of the title compound. [a] D = +6.5° (methanol). Amine is converted to oxalate and recrystallized from methanol / diethyl ether: M.p. 183-185°C. MS m / z (relative intensity, 70 eV) 229 (M+, 1), 199 (86), 187 (bp), 157 (49), 133 (42).
Embodiment 2
[0159] (+)-3-(3-chloro-2-fluorophenyl)-1-ethyl-3-methoxypyrrolidine
[0160] Add iodoethane (0.13ml, 1.63mmol) to (+)-3-(3-chloro-2-fluorophenyl)-3-methoxypyrrolidine (0.25g, 1.08mmol) and triethylamine (0.305ml, 2.17mmol) in tetrahydrofuran (20ml) and the solution was stirred at ambient temperature for 26 hours. Water (20ml) was added, the aqueous phase was extracted with EtOAc (2x50ml), and the combined organic phase was dried (Na 2 SO 4 ), to evaporate the solvent. By HPLC in waters OBD C18, 5μm(MeOH / 33mM NH 3 , 20:80 to 65:35) to obtain 0.106g (38%) of the title compound. [a] D = +16.5° (methanol). Amine is converted to fumarate and recrystallized from 2-propan / diisopropanol ether: M.P. 131-133°C. MS m / z (relative intensity, 70 eV) 257 (M+, 9), 242 (bp), 227 (44), 157 (44), 71 (89).
Embodiment 3
[0162] (-)-3-(3-Chloro-2-fluorophenyl)-3-methoxypyrrolidine
[0163] Prepared according to Example 1. Combine (-)-1-benzyl-3-(3-chloro-2-fluorophenyl)-3-methoxypyrrolidine (0.82g, 2.56mmol), 1,2-dichloroethane (10ml ), 1-chloroethyl chloroformate (1.12ml, 10.25mmol) was refluxed for 2 hours, and methanol (20ml) was added and refluxed for 1 hour. Purified by HPLC on waters OBD C18, 5 μm (MeOH / 33mM NH3, 20:80 to 50:50) to obtain the title compound (0.38g, 65%). [a] D = -7.1° (methanol). Amine is converted to oxalate and recrystallized from methanol / diethyl ether: M.p. 183-185°C. MS m / z (relative intensity, 70 eV) 229 (M+, 1), 199 (87), 187 (bp), 157 (55), 133 (51).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com