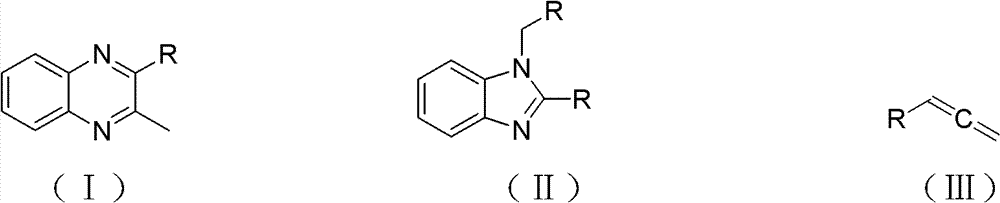
Method for preparing quinoxaline compounds and benzimidazole compounds
A technology of quinoxillin and benzimidazole, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of unobtainable raw materials, complicated operation and the like, and achieves the effects of convenient operation, high product purity and wide industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
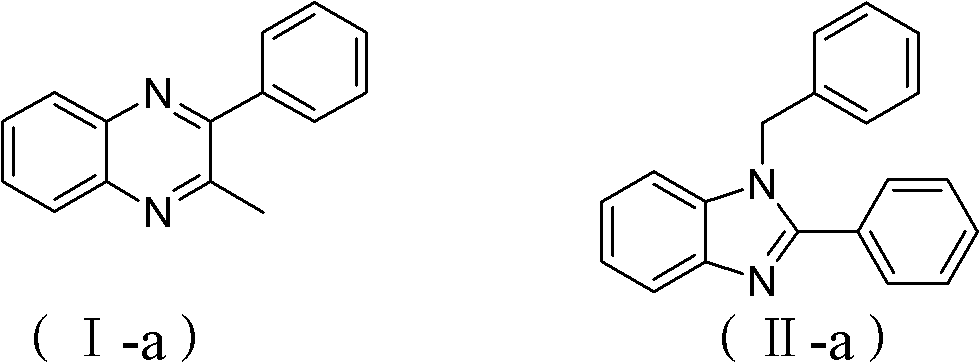
[0020] Embodiment 1 quinoxacillin compound I-a, benzimidazole compound II-a
[0021] With 58mg (0.5mmol) phenylpropadiene, 4.94mg (0.01mmol) triphenylphosphine gold chloride ((PPh) 3 AuCl), 7.8mg (0.04mmol) of silver tetrafluoroborate, 2uL (0.005mmol) of 98% concentrated sulfuric acid and 150uL (8.3mmol) of water were mixed in 1mL of dioxane, reacted at 60°C for 24 hours, and then added 37.8mg (0.35mmol) o-phenylenediamine, 9.0mg (0.1mmol) oxalic acid, reflux reaction at 80°C for 4 hours, TLC follow-up detection, after the reaction was completed, extracted with ethyl acetate (50mL×3), dried over anhydrous sodium sulfate, and filtered , concentrated, separated by silica gel column chromatography (the height of the chromatographic column is 20cm, and the diameter is 1cm; the elution flow rate is 1mL / min) (eluent: V 石油醚 :V 乙酸乙酯 =5:1), TLC (developing solvent: V 石油醚 :V 乙酸乙酯 = 5:1) Tracking detection collection R f For the eluent with a value of 0.6 to 0.65, the eluent was dis...
Embodiment 2
[0026] Embodiment 2 Quinocillin compound I-a, benzimidazole compound II-a
[0027]Dioxane was replaced by tetrahydrofuran, and other operations were the same as in Example 1 to obtain 9.53 mg of 2-methyl-3-phenylquinoxicillin (I-a), with a yield of 8.7%; 1-benzyl-2-phenyl Benzimidazole (II-a) 6.58 mg, yield 9.3%.
Embodiment 3
[0029] Dioxane was replaced with toluene, and other operations were the same as in Example 1 to obtain 20.0 mg of 2-methyl-3-phenylquinoxacillin (I-a), with a yield of 18.2%, 1-benzyl-2-phenyl Benzimidazole (II-a) 19.5 mg, yield 27.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com