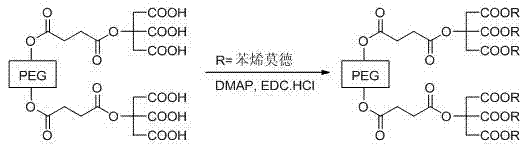
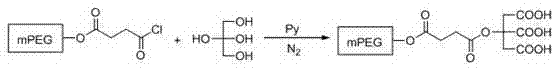
PEG/mPEG (Polyethylene Glycol) multi-carboxyl chemical modifying agent connected with citric acid, preparation method and application thereof in modification of toluylene compound
A chemical modifier and stilbene technology, which is applied in the directions of non-active ingredients, such as medical preparations, anti-inflammatory agents, anti-toxic agents, etc., to achieve the effects of simple preparation method, improved and improved drug performance, and increased drug loading capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment includes the following steps:
[0041] ①Preparation of PEG2000 carboxylic acid derivatives
[0042] The reaction formula is as follows:
[0043]
[0044] Take 40 g (20 mmol) of dry PEG, dissolve it in 160 mL of chloroform, add 5.0 g (50 mmol) of succinic anhydride, stir well, add 3 mL of pyridine (Py), heat to reflux, react for 36 h, evaporate the solvent, The residue was washed with 80 mL saturated NaHCO 3The solution was dissolved, extracted with ethyl acetate (20 mL×3), cooled the water phase to 0-5 °C, adjusted the pH to 2-3 with 1 mol / L hydrochloric acid, stirred for 20 min, extracted with dichloromethane (25 mL×4), combined The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized from anhydrous ether to obtain 37.4 g of PEG2000-succinic acid as a white solid, with a yield of 85.0%;
[0045] ②Synthesis of PEG2000-acyl chloride
[0046] The reaction formula...
Embodiment 2
[0059] This embodiment is carried out according to the following sequence of steps:
[0060] ①The preparation of PEG2000 carboxylic acid derivatives, PEG2000-acyl chlorides, and PEG2000 chemical modifiers connected with citric acid are the same as steps ①~③ in Example 1;
[0061] ②Preparation of PEG2000-succinyl-citric acid-oxidized resveratrol prodrug
[0062] The reaction formula is as follows:
[0063]
[0064] Take 0.5 g (0.20 mmol) of PEG2000 multi-carboxyl chemical modifier linked with citric acid, dissolve it in 5 mL of dichloromethane, add a DMF solution of oxidized resveratrol (0.35 g of oxidized resveratrol dissolved in 6 mL of DMF prepared in ), stirred for 30 min, added EDC.HCl 0.27 g (1.42 mmol), DMAP 1.73 g (1.42 mmol), and reacted at room temperature for 24 h. Acyl-citrate-oxidized resveratrol prodrug 0.30 g, yield 38.0%.
[0065] Since the most commonly used molecular weight of PEG / mPEG as a drug carrier is 2000-75000, this embodiment also replaces the a...
Embodiment 3
[0067] This embodiment is carried out according to the following sequence of steps:
[0068] ①The preparation of PEG2000 carboxylic acid derivatives, the preparation of PEG2000-acyl chloride, the preparation of the chemical modifier of PEG2000 multi-carboxyl group connected with citric acid, the method is the same as the steps ①~③ in Example 1 respectively;
[0069] ② Preparation of PEG2000-succinyl-citric acid-picetanol
[0070] The reaction formula is as follows:
[0071]
[0072] Take 0.5 g (0.20 mmol) of PEG2000 multi-carboxyl chemical modifier linked with citric acid, dissolve it in 5 mL of dichloromethane, add the DMF solution of piceatanol (0.35 g piceatanol dissolved in 6 mL of DMF) obtained), stirred for 30 min, added EDC.HCl 0.27 g (1.42 mmol), DMAP 1.73 g (1.42 mmol), and reacted at room temperature for 24 h. - Citric acid-picetanol prodrug 0.27 g, yield 34.0%.
[0073] Since the most commonly used molecular weight of PEG / mPEG as a drug carrier is 2000-75000, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com