Pyrrolidine borate dipeptidyl peptidase inhibitor and pharmaceutical composition thereof
A composition and drug technology, applied in the field of pyrrolidine borate DDP-IV inhibitors, can solve the problems that DDP-IV inhibitors have not yet been studied on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
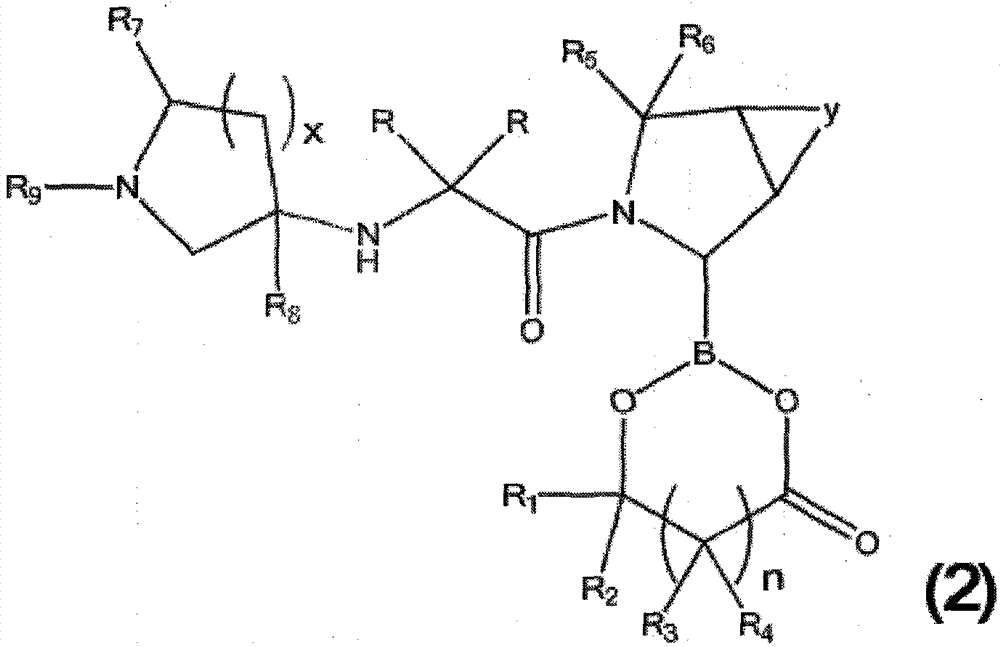
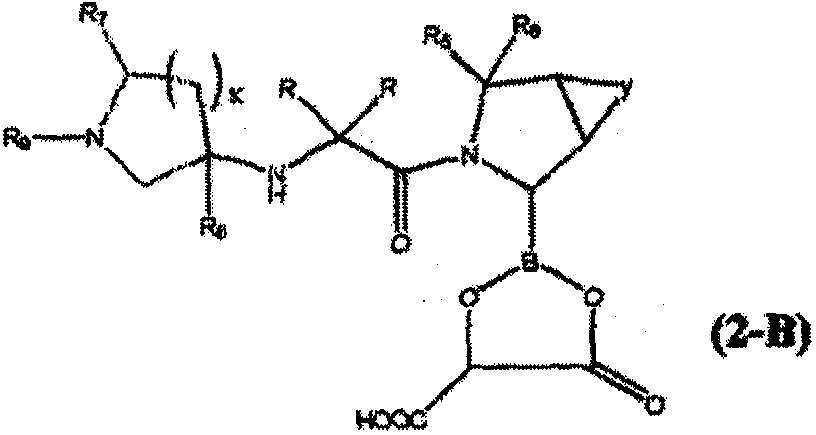
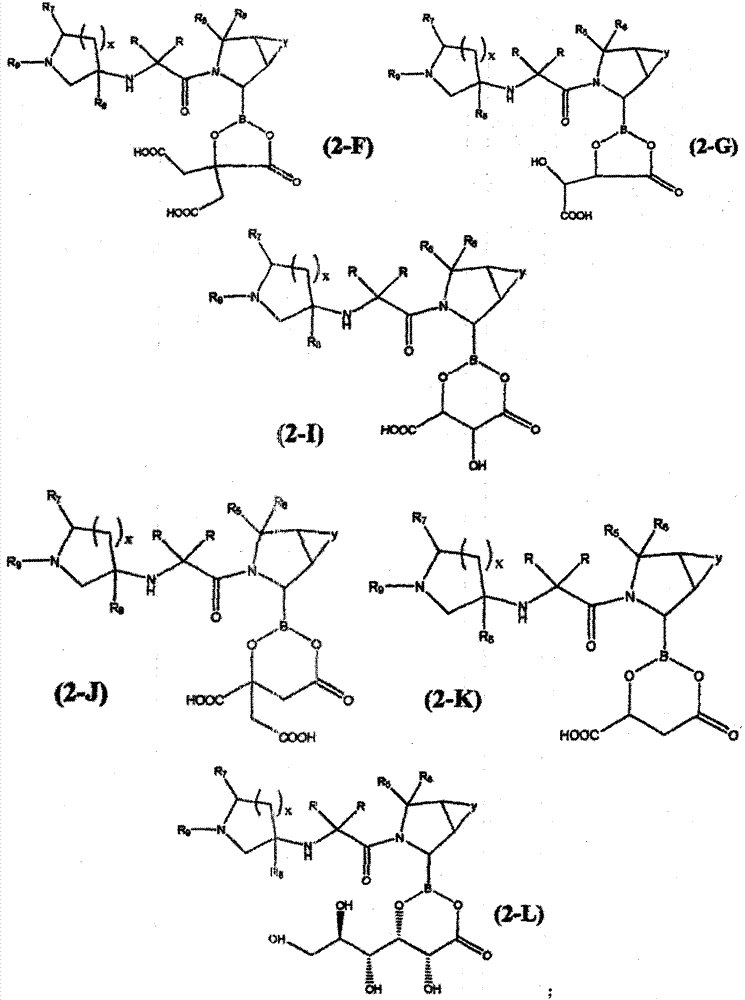
[0258] In a specific preferred technical scheme, the invention provides a method for preparing a borate compound containing chemical formula (1) by freeze-drying a mixture of pyrrolidine boronic acid compound and sugar, sugar alcohol or polyribose . The method comprises: (a) preparing a mixture comprising (i) water, (ii) a pyrrolidine boronic acid compound, and (iii) a sugar, sugar alcohol, polymeric alcohol or hydroxycarboxylic acid; (b) lyophilizing the mixture dry.
[0259] In a more preferred specific technical scheme, the invention provides the preparation of pyrrolidine boronic acid containing chemical formula (2) by spray-drying a mixture of pyrrolidine boronic acid compound and sugar, sugar alcohol, polymeric alcohol or hydroxycarboxylic acid Method for ester synthesis. The method comprises: (a) preparing a mixture comprising (i) water, (ii) a pyrrolidine boronic acid compound, and (iii) a sugar, sugar alcohol, polymeric alcohol or hydroxycarboxylic acid; (b) lyophil...
Embodiment 1
[0329] Example 1: Preparation of [(2R)-1-{N-[(3R)-pyrrolidin-3-yl]glycyl}pyrrolidin-2-yl]boronic acid compound e
[0330] The synthesis of compound e is completed according to Synthesis Scheme 2 (Scheme-2), involving three main steps. The first step is to combine compound a with compound b to generate compound c. This process undergoes a deprotection from the protecting group at the amino terminal of compound d the process of. Compound d is hydrolyzed under acidic conditions to generate boronic acid compound e.
[0331] Pinanediol borate (25mmol) added to compound a (25mmol) dissolved in 50mL of dichloromethane at an internal temperature lower than 5°C, and then added with DIPEA (13ml 75mmol) was added dropwise over 2 hours. Stir the reaction mixture at 0-5°C for at least 4 hours, then raise the temperature to 15-25°C, and continue stirring for at least 3-5 hours until the reaction is complete. Dilute the compound with 90 ml of ethyl acetate (EtOAc) and wash with Wash once ...
Embodiment 2
[0336] Example 2: Preparation of [(2R)-1-{N-[(3R)-pyrrolidin-3-yl]glycyl}pyrrolidin-2-yl]D-mannitol borate to [(2R) )-1-{N-[(3R)-pyrrolidin-3-yl]glycyl}pyrrolidin-2-yl]boronic acid (1.75g, 7.5mmol) in water and ethanol (25ml, 90:10, v / v) D-mannitol (10 mmol) was added to the solution. The suspension was heated to about 45°C for 5 minutes to completely dissolve the compound. Stir to dissolve the compound and then cool to room temperature, and filter the solution through a 0.45Am nylon membrane. This solution was frozen at minus 45°C shelf temperature by lyophilization. After one hour the vacuum was reduced. Let the temperature rise slowly to minus 35C and hold at minus 35C to evaporate all the freeze (about 40 hours). Turn off the rack temperature control and allow the temperature to gradually rise to 0°C. The secondary drying process is to allow the shelf temperature to rise to 25 degrees Celsius in three gradients within 4 hours. Then keep the shelf temperature at 25 d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com