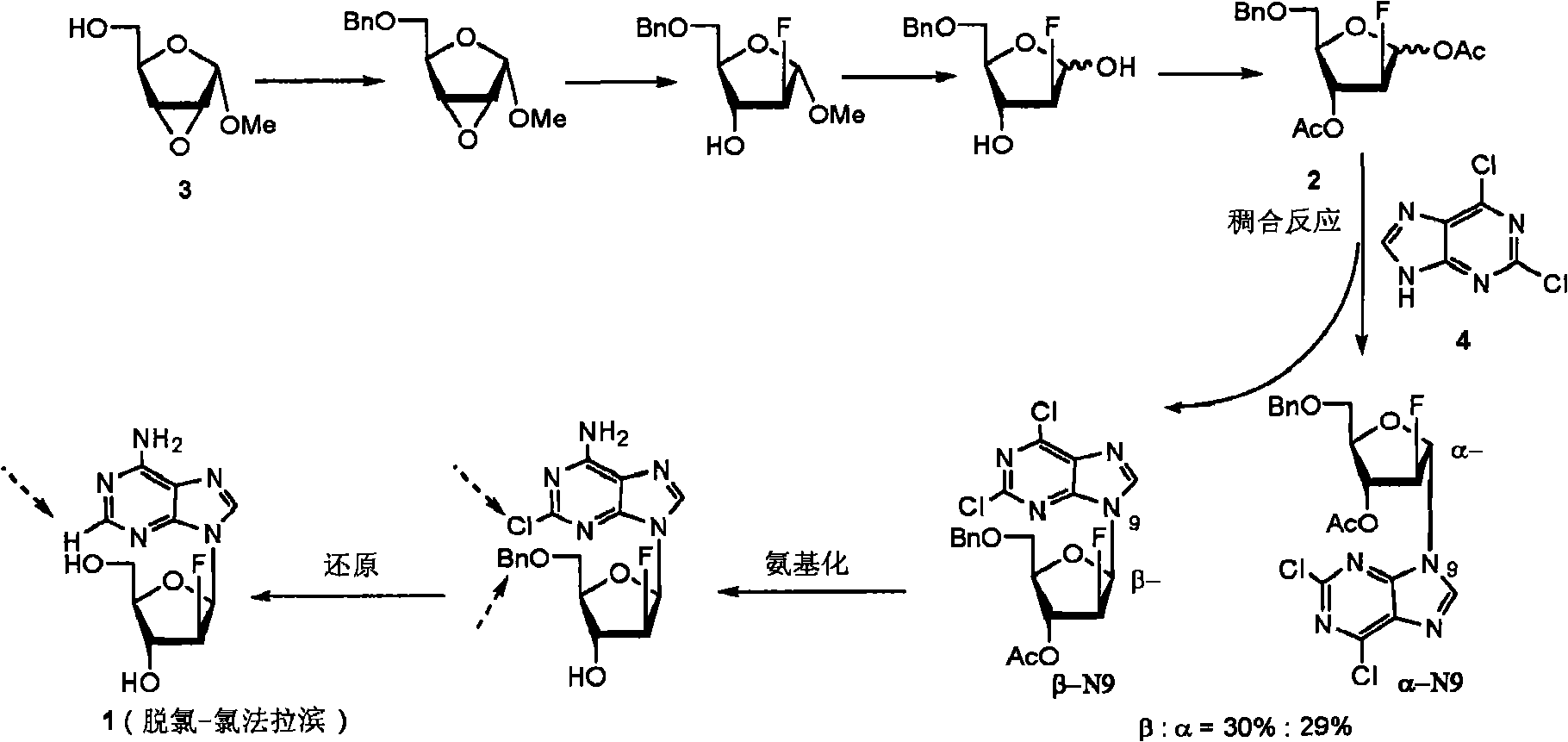
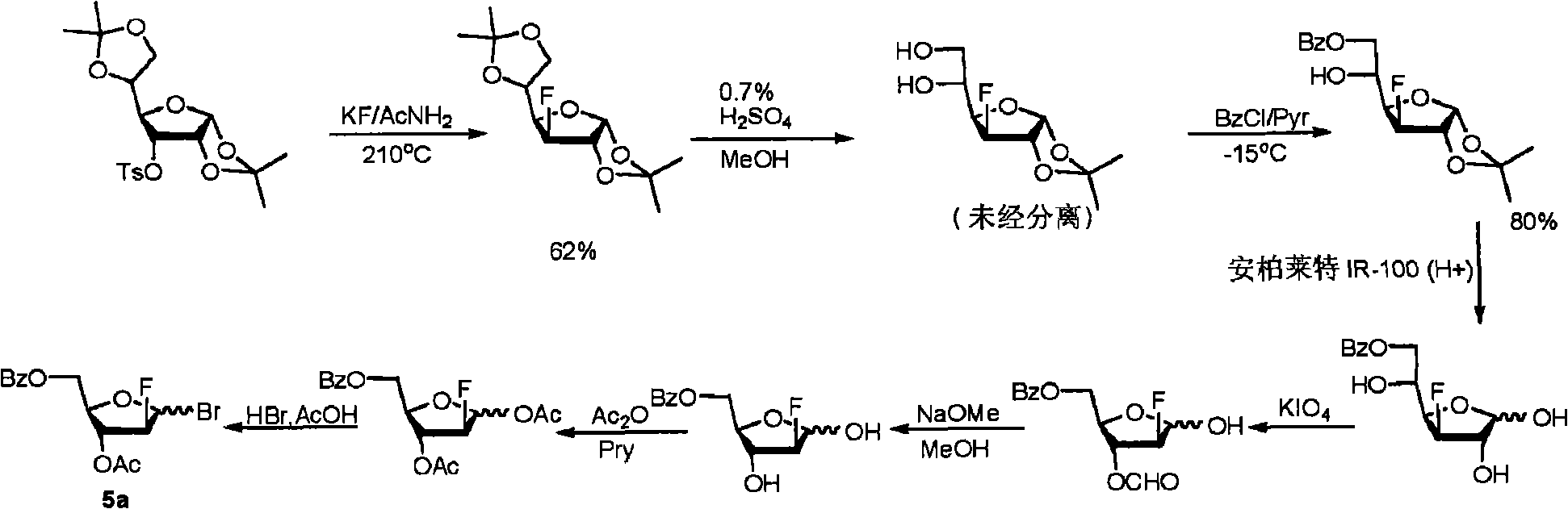
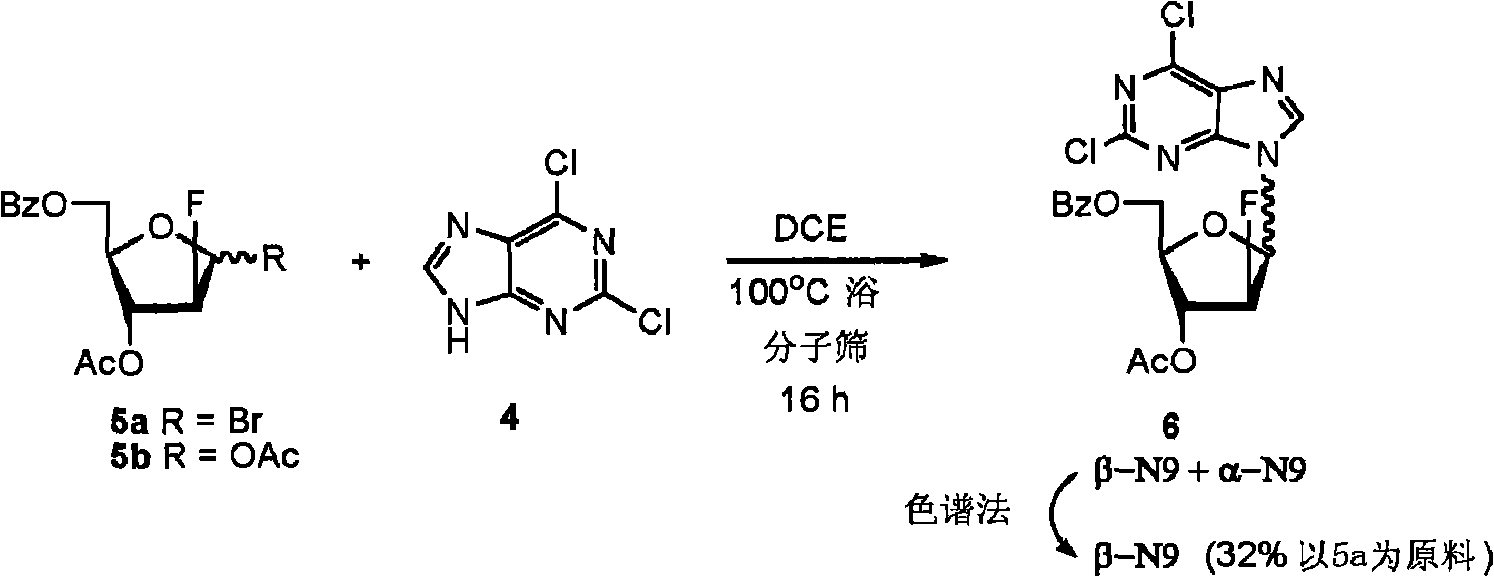
Preparation of 2-chloro-9-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-adenine
一种氯腺嘌呤、氯法拉滨的技术,应用在糖衍生物制备、糖衍生物、糖衍生物等方向,能够解决未表明大规模制造氯法拉滨等问题,达到降低负担、提高产率、工艺方法有效的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0107] Embodiments of the inventive method
[0108]
[0109] Preparation of 2',3',5'-tri-O-benzoyl-2-chloroadenosine (CF-IM1)
[0110] Step 1a - Silanization :
[0111] 2-Chloroadenine (CF-SM1(9), 50 g, 294.9 mmol, 1.0 eq.), MeCN (600 mL, 12P w.r.t. CF-SM1(9)) and BSTFA (227.5 g , 883.8mmol, 3.0eq.) into a flask equipped with mechanical stirring and a thermometer. The mixture was stirred and heated at reflux until the mixture became roughly clear (about 1 hour).
[0112] Step 1b - Wilbergen Glycosylation :
[0113] TfOH (8.77g, 58.5mmol, 0.2eq.) and 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (CF-SM2, 142.5g, 282.5 mmol, 0.95eq.) were sequentially added to the mixture prepared in step 1a. Subsequently, the mixture was stirred under reflux for 1 hour. The mixture was cooled to 20-35 °C, diluted with MTBE (500 mL), and washed with saturated NaHCO 3 (750 mL) for 0.5 to 1 hour. Evaporation of organic volatiles in vacuo at 40 to 50°C afforded CF-IM1: 1 H NMR (30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com