Medicine combination containing fusion protein for suppressing angiogenesis and application
A fusion protein and angiogenesis technology, which is applied in the direction of drug combination, non-active ingredient medical preparations, pharmaceutical formulas, etc., can solve the problems of reduced protein activity, poor stability of fusion protein, and decreased therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Stability study of the original formulation of 10mg / ml FP3 fusion protein in 3ml glass ampoule at 4°C
[0030] The prescription is as follows:
[0031] FP3 fusion protein 10mg / ml
[0032] Sodium succinate 10mM
[0033] Trehalose 9.0%
[0034] Tween 20 0.05%
[0035] Adjust the pH of the system to 6.0-6.5 with hydrochloric acid
[0036] After changing the protein stock solution, aseptically dispense it into 3ml glass ampoules, reserve samples at 4°C, and measure at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and December Samples, stability determined by SEC-HPLC. The results showed that the formulation could not effectively inhibit the formation of polymers, resulting in a decrease in product purity and a decrease in affinity with VEGF, which may induce an immune response after entering the body.
[0037] Table 1.10mg / ml FP3 fusion protein stability at 4°C
[0038] time (month)
Embodiment 2
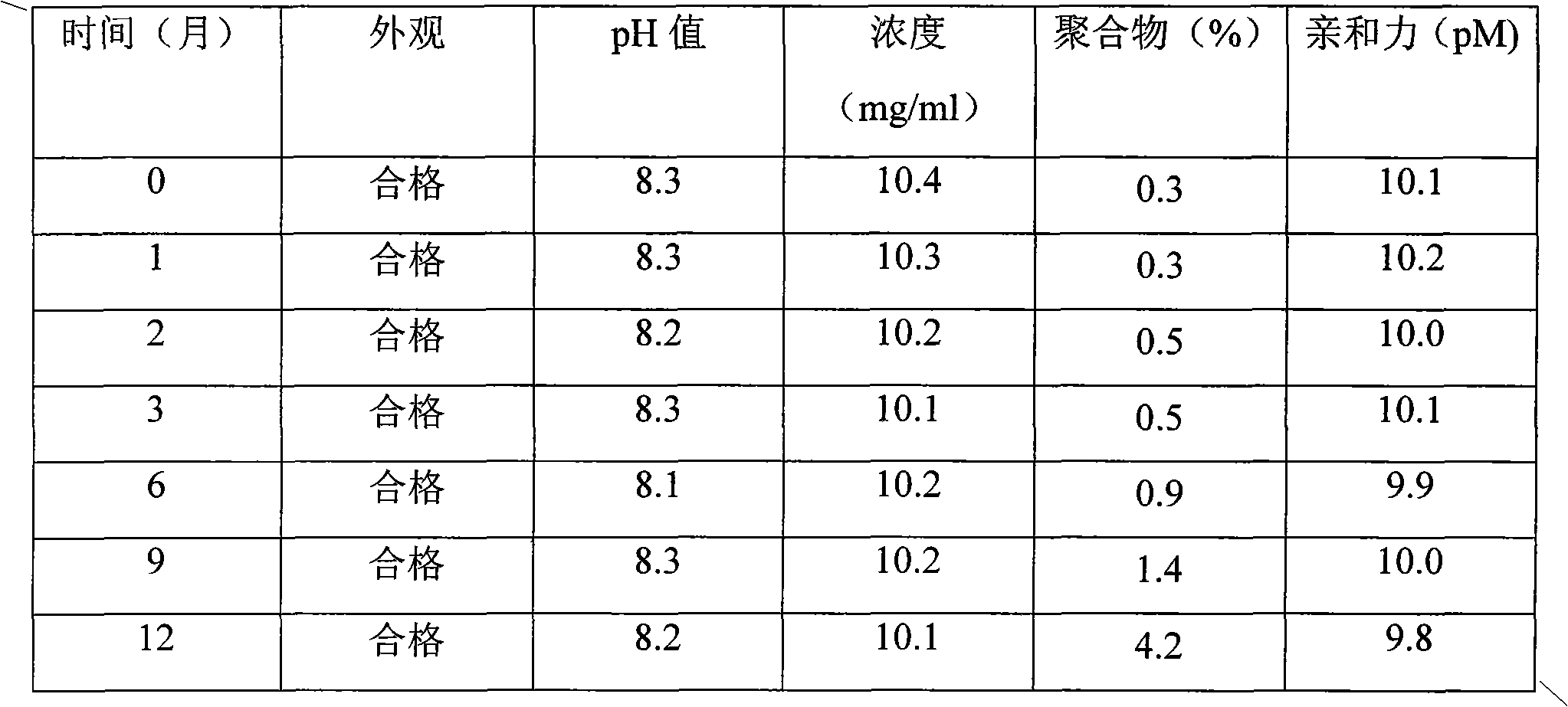
[0039] Example 2 Stability study of 10mg / ml FP3 fusion protein in 3ml glass ampoule at 4°C
[0040] FP3 fusion protein 10mg / ml
[0041]Disodium hydrogen phosphate 10mM
[0042] Sucrose 10%
[0044] Tween 20 0.05%
[0045] pH 7.5~8.3
[0046] After changing the protein stock solution, aseptically dispense it into 3ml glass ampoules, reserve samples at 4°C, and measure at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and December Samples, stability determined by SEC-HPLC. The results showed that the formulation could not effectively inhibit the formation of polymers, resulting in a decrease in product purity and a decrease in affinity with VEGF, which may induce an immune response after entering the body.
[0047] Table 2.10mg / ml FP3 fusion protein stability at 4°C
[0048] time (month)
Embodiment 3
[0049] Example 3 Stability study of 10mg / ml FP3 fusion protein in 3ml glass ampoule at 4°C
[0050] FP3 fusion protein 10mg / ml
[0051] Citric acid 5mM
[0052] Sucrose 8.0%
[0053] Tween 20 0.05%
[0054] pH 7.5~8.3
[0055] After changing the protein stock solution, aseptically dispense it into 3ml glass ampoules, reserve samples at 4°C, and measure at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and December Samples, stability determined by SEC-HPLC. The results showed that the formulation could not effectively inhibit the formation of polymers, resulting in a decrease in product purity and a decrease in affinity with VEGF, which may induce an immune response after entering the body.
[0056] Table 3.10mg / ml FP3 fusion protein stability at 4°C
[0057] time (month)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com