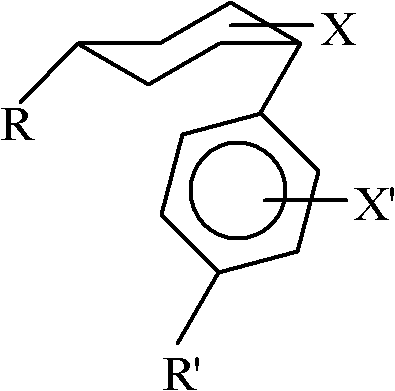
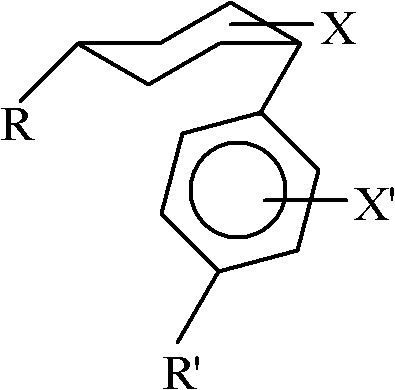
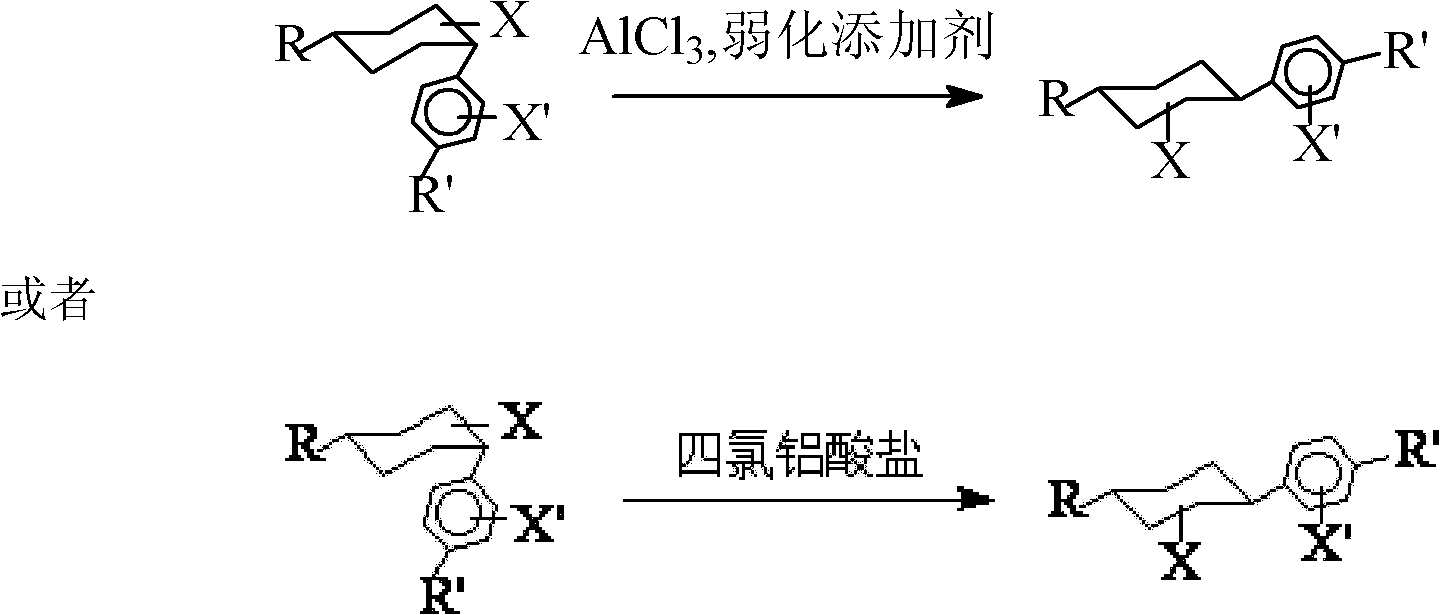
Method for converting cis-substituted cyclohexyl in organic molecules into trans-substituted cyclohexyl
A cis, organic technology, applied in organic chemistry, organic isomerization, chemical instruments and methods, etc., can solve the problems of limited selection of passivation catalysts, large amount of transposition catalysts, low temperature tolerance, etc., to achieve inhibition The effect is good, the transposition method is simple and easy, and the effect of long reaction time range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0058] Synthetic Example 1: Transposition of 4-(4'-propylcyclohexyl)cyclohexylbenzene (cis: trans = 70:30 in raw materials).
[0059]
Embodiment 1
[0061] The synthetic route of synthetic example 1 is adopted. Anhydrous aluminum trichloride-triethylamine hydrochloride mixture is used as a catalyst; the solvent is petroleum ether, and the temperature is 5-10°C.
[0062] Experimental operation:
[0063] In a 100ml three-necked flask, first add 0.1mol of triethylamine and 60ml of petroleum ether (90-120°C). Then accurately add 0.105 mol of hydrochloric acid aqueous solution dropwise under stirring. Stir at room temperature for 30 minutes after dropping. Heat under stirring until the petroleum ether refluxes, and use a water separator to clean the water brought out by the reflux. After no water comes out, cool down to room temperature, and add 0.105 mol of anhydrous aluminum trichloride while stirring. Stirring was continued at room temperature for 1 hour after the addition was complete. Stirring was stopped, and it was found that all the solids were dissolved, and the reaction solution was divided into two layers: the u...
Embodiment 2
[0071] The synthetic route of synthetic example 1 is adopted. Anhydrous aluminum trichloride-nitrobenzene mixture is used as a catalyst; the solvent is dichloromethane, and the temperature is 10-15°C.
[0072] Experimental operation:
[0073] The cis: trans = 70:30 raw material samples were dried, and the water and polar solvents were completely removed for later use.
[0074] Into a 100ml three-necked flask, add 60ml of dichloromethane and 0.50g of nitrobenzene. Add 0.5 g of anhydrous aluminum trichloride quickly at room temperature. After the addition, maintain room temperature and argon protection, and stir for more than 1 hour. Stir and cool down to the temperature of the reaction solution at 10-15° C., and quickly add 30 g of the above-mentioned spare raw material samples under the protection of argon. Maintain stirring and liquid temperature at 10-15°C. About 5ml of the reaction solution was taken out at regular intervals, washed with 20ml of 5% hydrochloric acid, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com