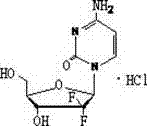
Synthesis method of gemcitabine hydrochloride
A technology of gemcitabine hydrochloride and its synthetic method, which is applied in the field of synthesis, can solve the problems of high cost, easy transformation of configuration, unfavorable batch production, etc., and achieve the effect of easy operation and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment
[0033] Embodiment: Synthesize gemcitabine hydrochloride according to the following steps
[0034] Step 1: Synthesis of GH-2: Add ethane (10L), propane (10L), D-mannitol (10KG, 54.9mol), anhydrous tin dichloride (10g, 0.05mol) into the reaction pot ; Heating to reflux, the reaction solution was clarified after 1 hour, and continued to react for 0.5 hours; cooled to 25°C, added pyridine (13ml), acetic anhydride (7ml), and stirred for 10 minutes; the solvent was evaporated under reduced pressure, and the internal temperature was controlled at 70 Below ℃; After evaporating to dryness, add dichloromethane to the residual liquid, stir for 30 minutes, and let it stand overnight to obtain GH-2 solution; the next day, suction filter, and the filtrate is pumped into the GH-3 reaction pot;
[0035] ;
[0036] Step 2: Synthesis of GH-3: Heat the GH-2 (6kg, 22.9mol) solution to 20°C, add sodium periodate (12.5kg, 58.6mol) under stirring; slowly add water dropwise, dropwise for half an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com