Bacterial compositions for prophylaxis and treatment of degenerative disease
A composition and bacterial technology, applied in the direction of bacteria, metabolic diseases, blood diseases, etc., can solve problems such as side effects, physical discomfort, and flushing of the whole body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2-
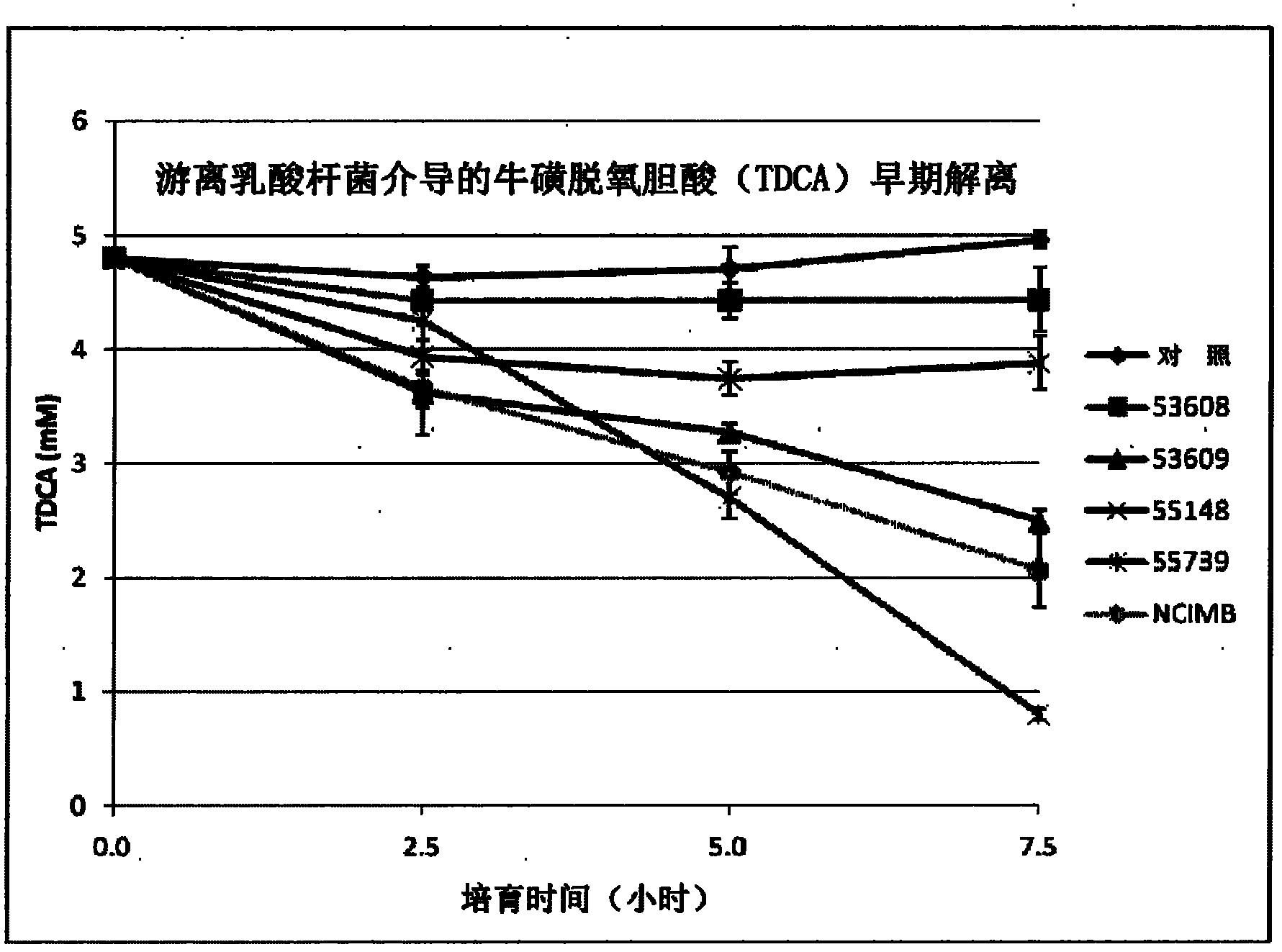
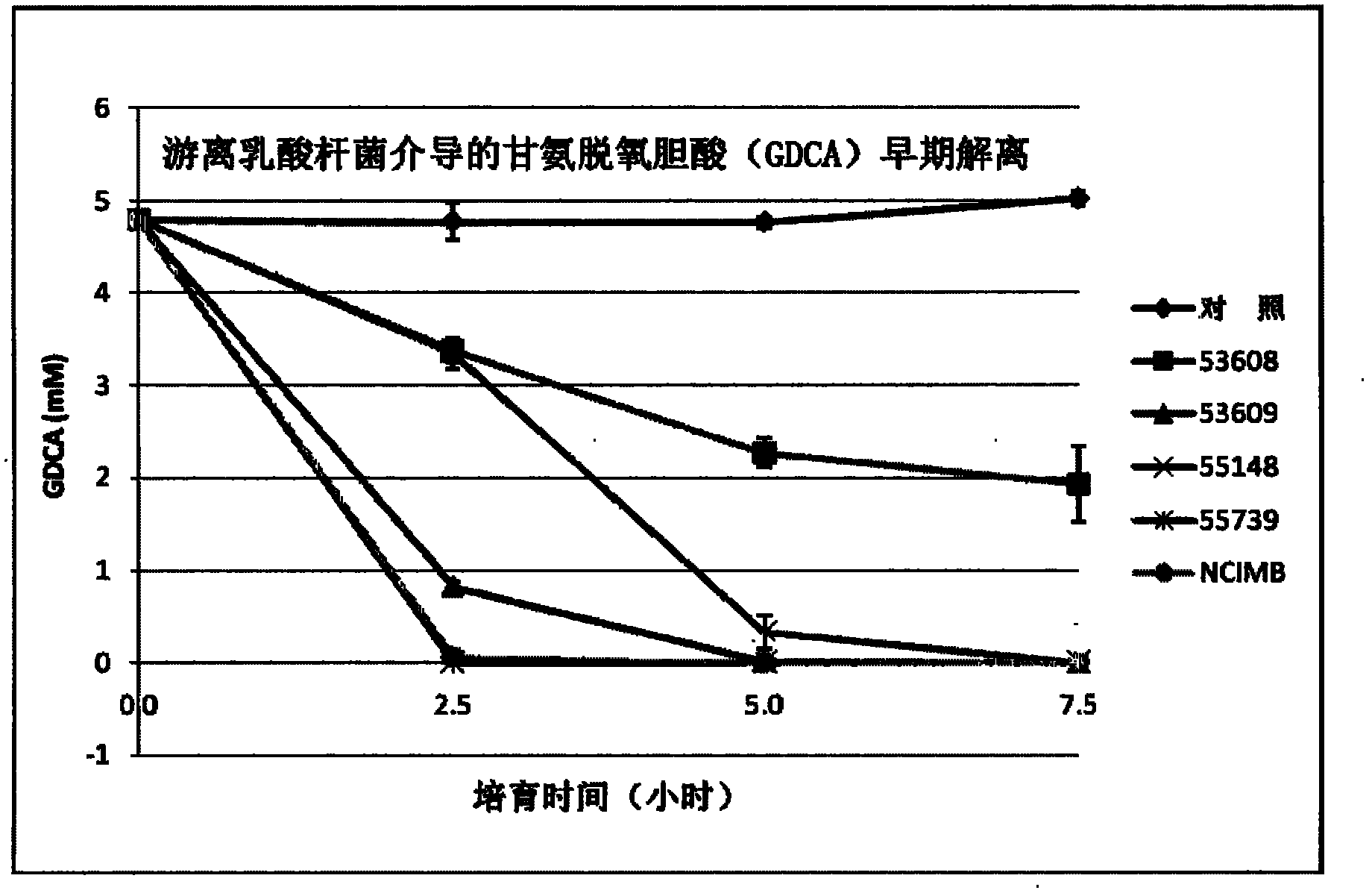
[0192] Example 2 - High BSH Activity
[0193] General Materials and Methods
[0194] Bacterial inoculation and growth: Glycerol-frozen bacterial stocks were picked with sterile wooden sticks on MRS agar plates. Incubate overnight under anaerobic conditions at 37°C. Under aseptic conditions, pick up the L. reuteri NCIMB 701359 monoclonal colony with a metal ring and transfer it to a tube containing 10 mL of MRS. Incubation overnight at 37°C is convenient for experiments.
[0195] Microcapsule encapsulation of L. reuteri NCIMB 701359: Microcapsules were prepared with 200 μm orifice, 8% cells and 1.75% concentration of sodium alginate. The embedding process is as follows: First, the CaCl 2Discharge the sodium alginate microspheres; second, wash the sodium alginate microspheres with 0.85% (w / v) NaCl for 10 min; third, embed the sodium alginate microspheres with 0.1% (w / v) ε-PLL for 20 min; Fourth, wash sodium alginate-PLL microcapsules with 0.85% (w / v) NaCl for 10 min; fif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com