Differentiation Of Human Embryonic Stem Cells
A technology of pluripotent stem cells and cells, applied in the direction of embryonic cells, artificial cell constructs, artificially induced pluripotent cells, etc., can solve the problem of reducing the ability of insulin-expressing cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
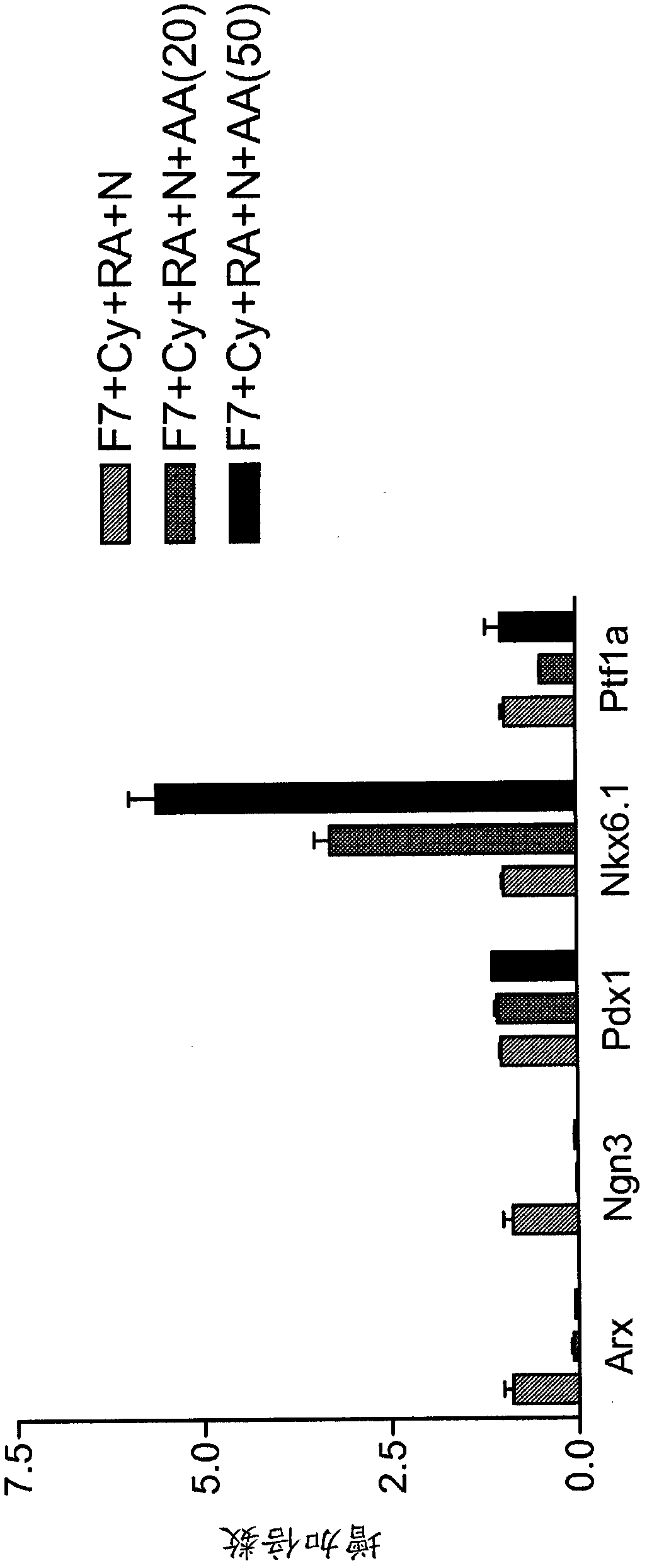
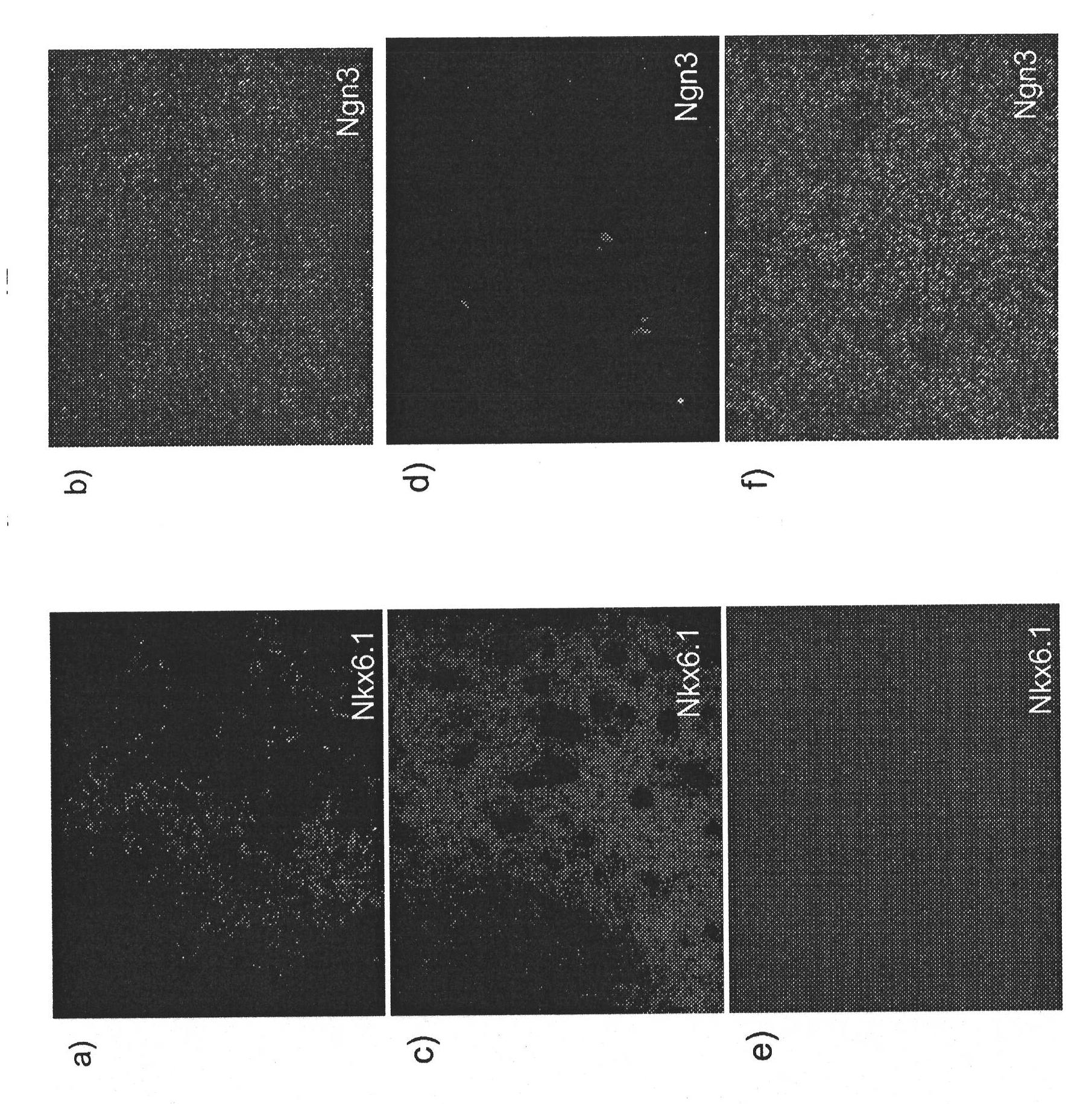
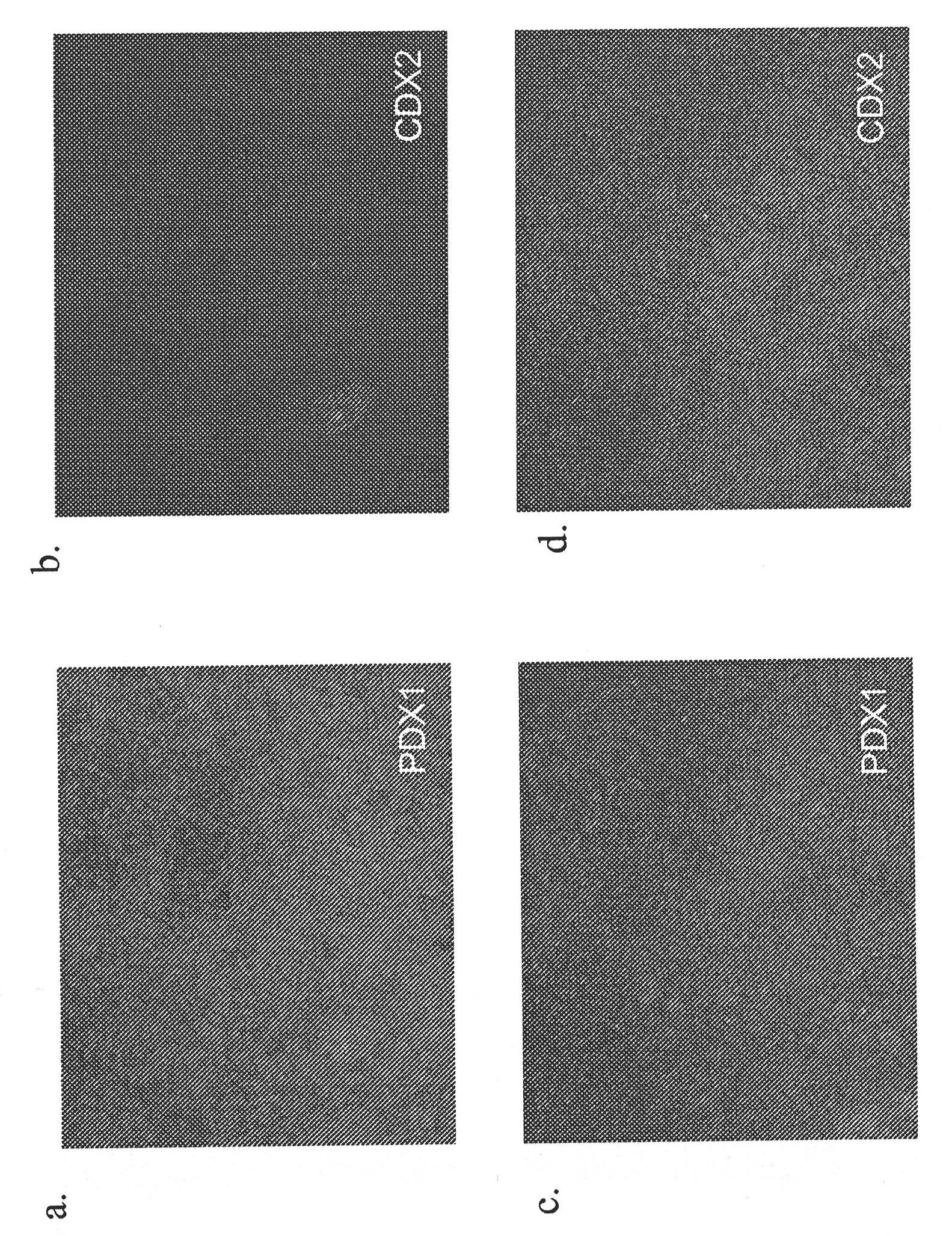
[0158] Human pluripotent stem cells express markers characteristic of the pancreatic endoderm lineage (Table 1 up to PDX1, NKX6.1, but not CDX2 and NGN3) differentiation
[0159] This example demonstrates that activin A can be used in combination with noggin and retinoic acid to promote the upregulation of NKX6.1 expression. Briefly, cells of the human embryonic stem cell line H1 were incubated in MATRIGEL TM (1:30 dilution) coated plate and supplemented with 2% BSA, 100ng / ml activin A, 20ng / ml WNT-3a, 8ng / ml bFGF cultured on the RPMI medium for one day, and then supplemented with 2%BSA , 100ng / ml activin A, 8ng / ml bFGF in RPMI medium for two days (phase 1), then
[0160] a. Treated with DMEM / F12+2%BSA+50ng / ml FGF7 for three days (stage 2), then
[0161] b. Use DMEM-high glucose+1% B27+50ng / ml FGF7+0.25mM cyclopamine-KAAD+2mM retinoic acid (RA)+100ng / ml noggin+20ng / ml activin A or 50ng / ml Activin A treatment for four days (phase 3).
[0162] As a control, separate cell...
example 2
[0180] Expression of markers characteristic of the pancreatic endoderm lineage (co-expression of PDX1, NKX6.1, but Differentiation of cells that do not express CDX2 and NGN3) into pancreatic endocrine precursors
[0181] Previous studies have shown that cells expressing markers characteristic of the pancreatic endoderm lineage are more likely to give rise to glucagon-expressing cells than insulin-expressing cells when undergoing further differentiation. This may be partly due to the expression of NGN3 in pancreatic endoderm cells. The methods of the invention can generate a population of pancreatic endoderm cells that do not express NGN3 and therefore will be more likely to differentiate into insulin expressing cells. However, NGN3 expression is essential for the formation of pancreatic endocrine cells or pancreatic endocrine precursor cells (cells that can form, for example, glucagon expressing cells or insulin expressing cells). Thus, temporal regulation of NGN3 is impo...
example 3
[0193] Expression of markers characteristic of the pancreatic endoderm lineage (co-expression of PDX1, NKX6.1, but Differentiation of cells that do not express CDX2 and NGN3) into pancreatic endocrine cells
[0194] This example was designed to test the ability of cells expressing markers characteristic of the glandular endoderm lineage (coexpressing PDX1 and NKX6.1 but not CDX2 and NGN3) to further differentiate from pancreatic endocrine precursors into pancreatic endocrine cells.
[0195] Briefly, cells of the human embryonic stem cell line H1 were incubated in MATRIGEL containing RPMI medium + 2% BSA + 100ng / ml Activin A + 20ng / ml WNT-3a + 8ng / ml bFGF TM Coated plates (1:30 dilution) were cultured for one day, then treated with RPMI medium+2%BSA+100ng / ml activin A+8ng / ml bFGF for two days (stage 1), and then
[0196] a. Treated with DMEM / F12+2%BSA+50ng / ml FGF7 for three days (stage 2), then
[0197] b. Treated with DMEM-high glucose+1% B27+50ng / ml FGF7+0.25μM cyclopami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com