Shikonin derivative containing fluorine carboxylic acid ester and synthetic method and application thereof
A technology of shikonin carboxylic acid and fluorine-containing carboxylic acid, which is applied to the preparation of carboxylic acid esters, drug combinations, chemical instruments and methods, etc., and can solve problems such as water solubility, toxic and side effects, and restrictions on the possibility of shikonin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
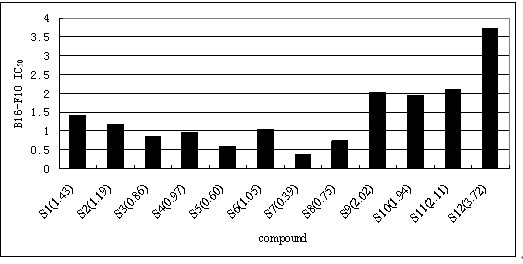
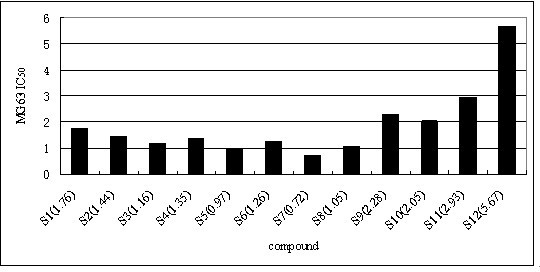
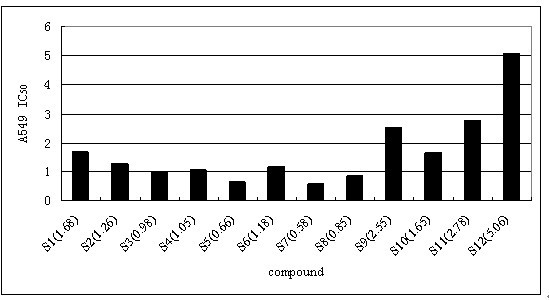
[0027] Example 1: The synthetic method of formula I class shikonin derivatives
[0028] Dissolve 50mmol of shikonin and 50mmol of the corresponding carboxylic acid in 20mL of anhydrous dichloromethane, and continue to add catalytic amounts of N,N-dicyclohexylcarbodiimide (DCC) and 4-Dimethylaminopyridine (DMAP), followed by TLC detection to generate corresponding ester derivatives. Add an appropriate amount of silica gel to concentrate the solvent under reduced pressure, and perform column chromatography with ethyl acetate:petroleum ether=1:4 to obtain the corresponding shikonin carboxylate derivatives.
[0029] The reaction takes 2,2-difluoroacetic acid as an example:
[0030]
[0031] Compound 1
[0032] Compounds 2-8 can be obtained in the same way.
[0033]
[0034] Compound 1 Compound 2 Compound 3 Compound 4
[0035]
[0036] Compound 5 Compound 6 Compound 7 Compound 8
[0037] The physicochemical data of the corresponding compounds are as follows:
[0038]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com