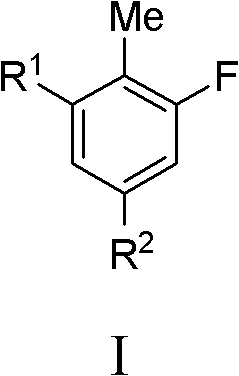
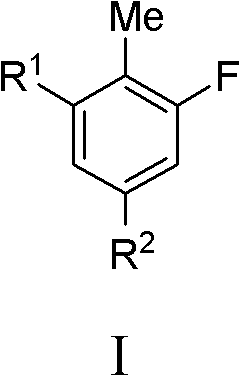
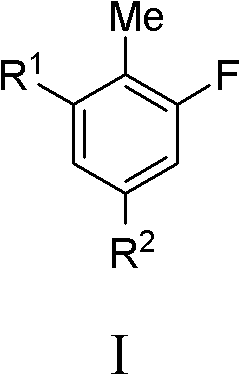
Fluoro-substituted compounds as kinase inhibitors and methods of use thereof
A compound and solvate technology, applied in the field of pharmaceutically active compounds and compound intermediates for the treatment of various tumor diseases, can solve problems such as genome instability and high cell division rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] Novel starting materials and / or intermediates and processes for their preparation are likewise subject of the present invention. In selected embodiments, such starting materials are used and reaction conditions are selected to obtain one or more desired compounds.
[0076] The starting materials of the present invention are known, commercially available or can be synthesized by methods known in the art or methods analogous thereto. Many starting materials may be prepared according to known methods, in particular using the methods described in the schemes and examples. During the synthesis of starting materials, functional groups are protected in some cases with suitable protecting groups when necessary.
[0077] Methods involving the use of protecting groups can be employed. In particular, if one or more functional groups (such as carboxyl, hydroxyl, amino or mercapto) are protected or need to be protected during the preparation of the compounds of the invention becau...
Embodiment 1-14
[0139] Embodiment 1-14 (method A)
[0140]
Embodiment 1
[0141] The preparation of embodiment 1.4-((4-methylpiperazin-1-yl) methyl) methyl benzoate
[0142]
[0143] Into a 250-mL three-neck round bottom flask was placed methyl 4-formylbenzoate (4.92 g, 30.0 mmol, 1.00 equiv) in CH 3 Solution in CN (100 mL), 1-methylpiperazine (3.6 mL, 1.30 equiv) and CF 3 COOH (2.5 mL, 1.10 equiv). The resulting solution was stirred at room temperature for 15 min, then NaBH was added in several portions 3 CN (3.78 g, 60.0 mmol, 2.00 equiv). The resulting solution was stirred overnight at room temperature. The reaction was terminated by adding 10 mL of water. The resulting mixture was concentrated in vacuo. The residue was applied to a silica gel column and eluted with dichloromethane / methanol (80:1). 4 g of methyl 4-((4-methylpiperazin-1-yl)methyl)benzoate were obtained as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com