Process for preparing gamma-valerolactone by utilizing iridium-pincer ligand complex catalyst
A pincer ligand and complex technology, which is applied in the field of preparing γ-valerolactone, can solve the problems of harsh reaction conditions, large amount of catalyst, and difficulty in large-scale production, and achieves mild reaction conditions, high yield, and high selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
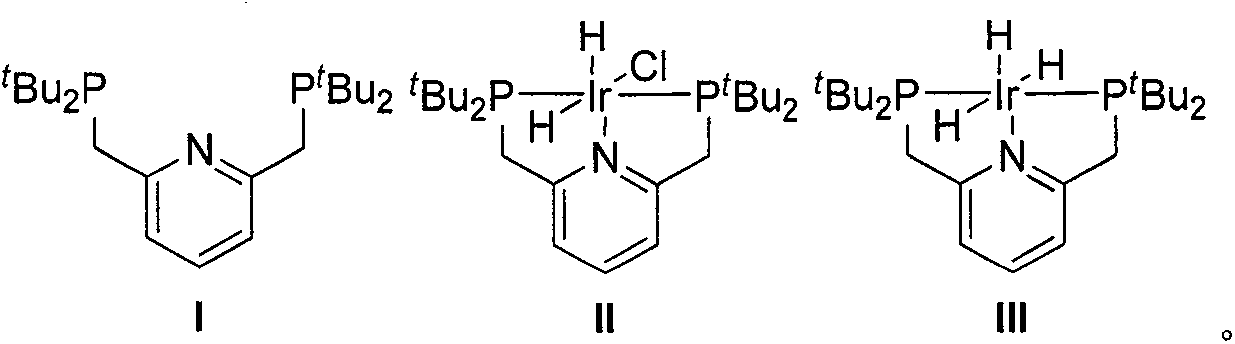
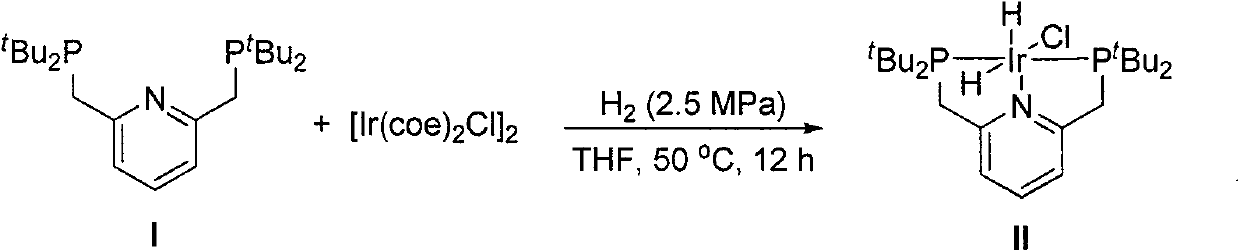
[0017] Preparation of Catalyst II
[0018]
[0019] Add tBu-PNp(I) (119 mg, 302 μmol) and [Ir(coe)2C1] 2 (90 mg, 100 μmol) to a dry and clean 10 mL Schlenk tube, replace the system with an argon atmosphere and add degassed tetrahydrofuran (5.5 mL), stirred and dissolved evenly, transferred the solution to an argon-protected high-pressure hydrogenation kettle, quickly replaced the argon three times, and adjusted the hydrogen pressure to 2.5MPa after replacing the hydrogen five times, heated the oil bath to 90°C, and stirred for 12 hours. . Cool to room temperature, transfer the system to another clean and dry 10mL Schlenk tube under the protection of argon, desolvate in vacuum to a residual volume of about 0.5mL, add degassed n-hexane (5mL), and precipitate a white precipitate. It was filtered under protection, and the filter cake was washed with degassed n-hexane (2×5 mL), and dried in vacuo to obtain product II (100 mg, 80%) as a white solid. 1 H NMR (400MHz, CDCl 3 )δ7...
Embodiment 2
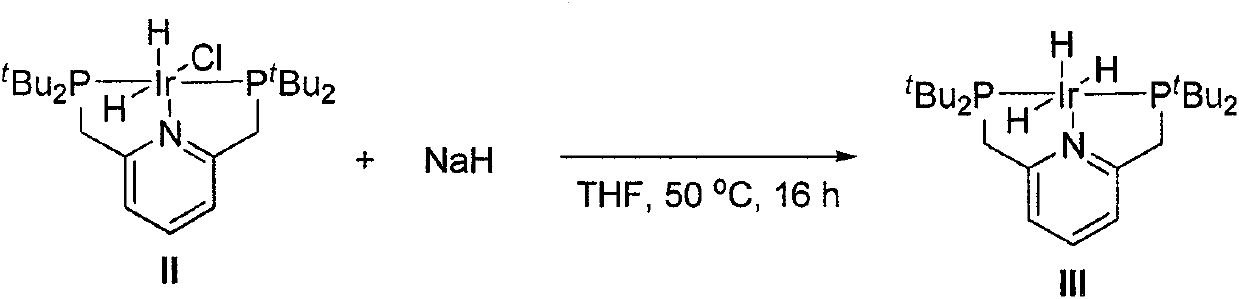
[0021] Preparation of Catalyst III
[0022]
[0023] Add ( t Bu-PNP)IrH 2 Cl(II) (125mg, 200μmol), sodium hydride (500mg, 20mmol), after replacing the system with argon atmosphere, add degassed tetrahydrofuran (6mL), heat the oil bath to 50°C and stir the reaction for 16 hours, then cool to room temperature After filtering under the protection of argon, the filtrate was transferred to another dry and clean 10mL Schlenk tube, and the volume of the system was vacuum precipitated to about 0.5mL, and degassed n-hexane (5mL) was added, and a brown solid was precipitated. The filter cake was washed with degassed n-hexane (2×5 mL) and dried in vacuo to obtain product III as a khaki solid (50 mg, 42%). 1 H NMR (400MHz, C 6 D. 6 )δ6.82(t, J=7.7Hz, 1H), 6.50(d, J=7.7Hz, 2H), 3.13(s, 4H), 1.42(t, J=6.4Hz, 36H), -10.12(td , J=15.3, 5.2Hz, 2H), -19.78(tt, J=14.8, 4.8Hz, 1H). 31 P NMR (162MHz, C 6 D. 6 )δ88.03(s). 1 H NMR (400MHz, CD 2 C1 2 )δ7.36(t, J=7.6Hz, 1H), 7.08(d, J=7....
Embodiment 3~8
[0025] Weigh [Ir(coe)2C1]2 (1.3mg, 1.5μmol) and fBu-PNP(I) (1.8mg, 4.5μmol) in a dry and clean 10mL Schlenk tube in the glove box, and replace the system with argon Add the corresponding degassed solvent (2 mL) after the atmosphere, heat the water bath to 50 ° C, stir and complex for 30 minutes, and then replace it with a hydrogen atmosphere (hydrogen balloon) and continue to stir and complex for 15 minutes. Add the pre-prepared levulinic acid (348.3mg, 3mmol) solution in the corresponding solvent (2mL) to the above catalyst solution, stir evenly and add the system to a weighing machine replaced by an argon atmosphere with 1.2 equivalents of hydroxide In the high-pressure hydrogenation kettle of potassium (85% purity) (230mg, 3.5mmol), after rapidly displacing the argon three times, rapidly displace the hydrogen five times, adjust the hydrogen pressure to 5Mpa, heat the oil bath to 100°C, stir the reaction (1250rpm) for 15 After one hour, the system was cooled to room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com