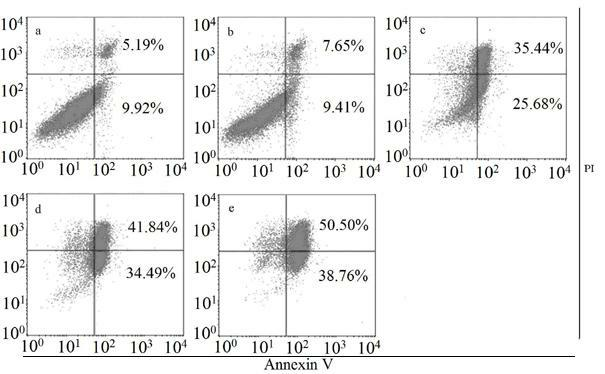
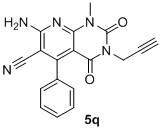
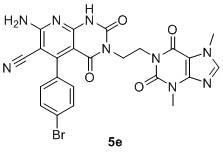
Pyridopyrimidine ketones derivative and application in preparing antitumor drugs thereof
An anti-tumor drug, the technology of pyrimidinone, applied in the field of chemical medicine, can solve the problem that there are very few reports on anti-tumor activity, and achieve a good effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] Accurately weigh the 4-amino-2,6-dihydridine 5g in the 250ml round-bottomed bottle, measured 4.17g of benzenedehyde, 2.47ml of CER, add 150ml of water, slowly hear to return, add 500mg tebac, return overnight to spend overnight overnight overnight overnight, return overnight to overnight to spend overnight and overnight overnight overnight to spend overnight and overnight overnight to spend overnight and overnight overnight to spend overnight and overnight overnight to spend overnight and overnight overnight to spend overnight and overnight overnight to spend overnight and overnight overnight overnight overnight.The next morning stopped the reaction, cooled to room temperature, and filtered 7.82g of gray -white solids, with an yield of 71.2%.
[0047] 1 H-NMR (400 MHz, DMSO-D 6 ) Δ 11.473 (s, 1H), 10.918 (s, 1H), 7.649 (s, 2H), 7.407 (m, 3H), 7.249 (m, 2H).
[0048] Example 2: 7-amino-2,4-dione-5- (4-chlorophenyl) -1,2,3,4-tetrahydrine [2,3-D] pyrimidine -6-腈 (3B)...
Embodiment 2
[0049]
[0050] The preparation method is the same as the embodiment 1, with a rate of 62.3%.
[0051] 1 H-NMR (400 MHz, DMSO-D 6 ) Δ 11.517 (s, 1H), 10.979 (s, 1H), 7.702 (s, 2H), 7.48 (D, J = 8.4Hz, 2H), 7.30 (D, J = 8Hz, 2H).
[0052] Example 3: 7-amino-2,4-dione-5- (3,4-di oxygen-phenyl) -1,2,3,4-tetrahydrine [2,3-D] pyrimidine-6-Preparation of 3 (3C)
Embodiment 3
[0053]
[0054] The preparation method is the same as the embodiment 1, with a rate of 66.1%.
[0055] 1 H-NMR (400 MHz, DMSO-D 6 ) Δ 11.433 (s, 1H), 10.911 (s, 1H), 7.601 (s, 2H), 6.98 (D, J = 8.4Hz, 1H), 6.89 (D, J = 1.6Hz, 1H), 6.811 (DD, J = 1.6Hz, J = 8Hz, 1H), 3.81 (s, 3H), 3.714 (s, 3H).
[0056] Example 4: 7-amino-2,4-dione-5- (3-methoxy-4-hydroxyl-phenyl) -1,2,3,4-tetrahydrine [2,3-D]Preparation of Pyrimidine-6-d (3D)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com