Method for detection of, or the risk of, bladder cancer
A bladder cancer, risk technology, applied in the direction of measuring devices, instruments, biomaterial analysis, etc., can solve the lack of accurate diagnosis of bladder cancer sensitivity and specificity of bladder cancer, has not yet reached the high sensitivity of urine cytology, can not correctly evaluate cells exam questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
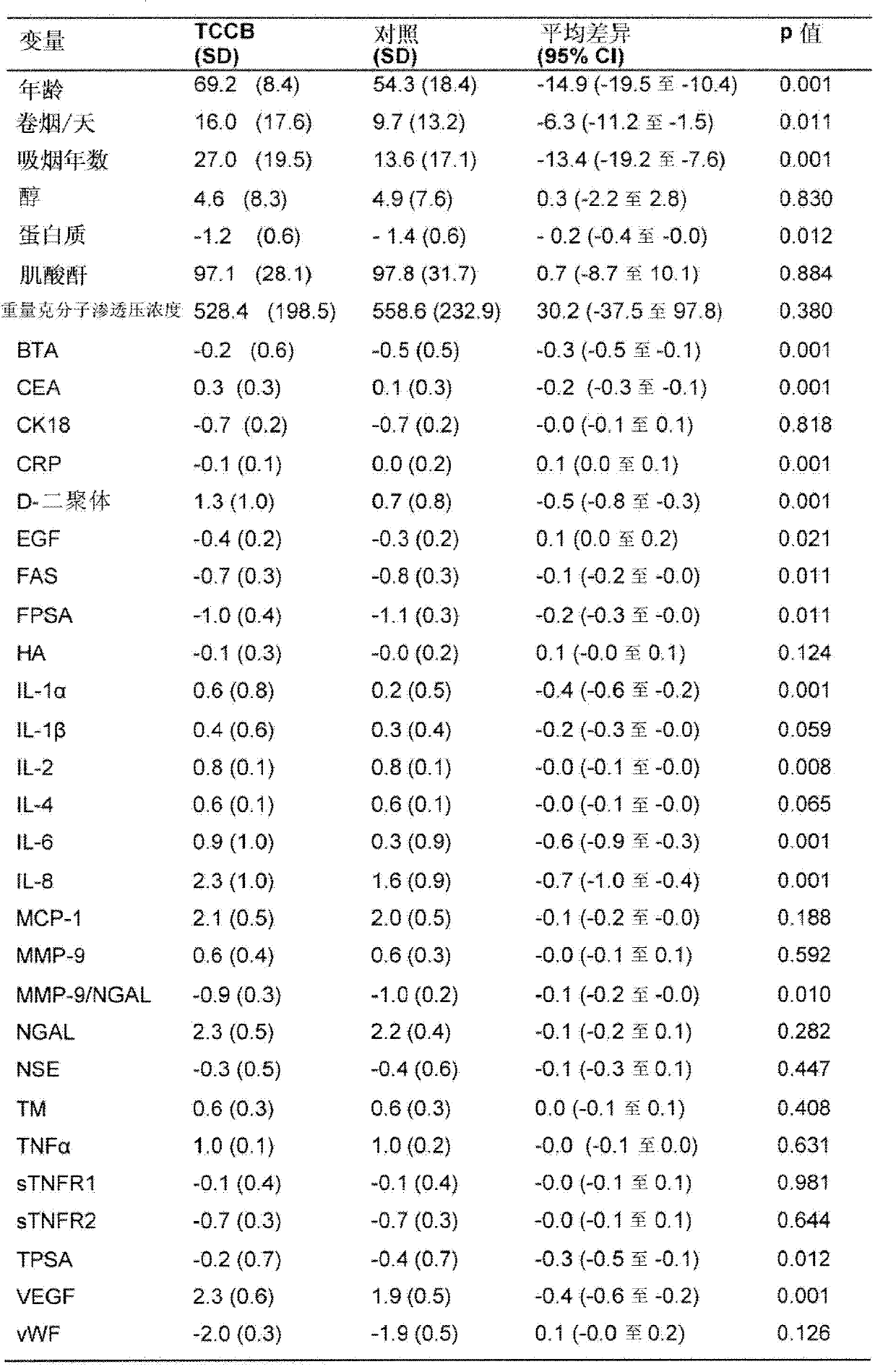
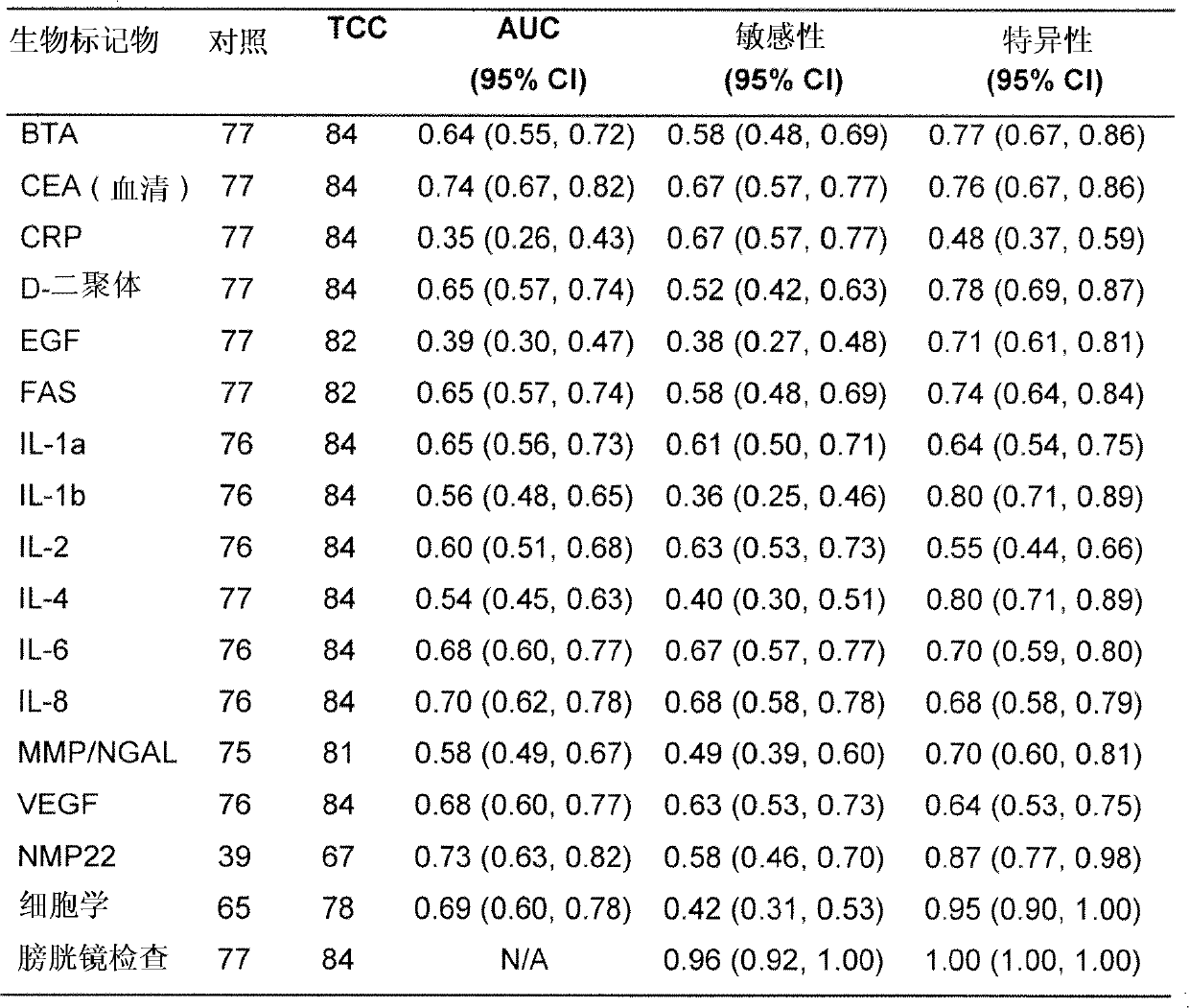
[0063] A pilot study was performed to determine which individual biomarkers were significantly altered in bladder cancer patients compared with controls, and which variables acted as risk factors for bladder cancer. Seventy-seven patients (55 males; 22 females) were designated as a control group based on negative cystoscopy and absence of symptoms of bladder cancer, followed by additional studies. Their final diagnoses were as follows: no diagnosis (n=36), benign prostatic hyperplasia (BPH) (n=13), stone (n=9), stone with inflammation (n=2), inflammation (n=4), Prostate cancer (n=3), renal cell carcinoma (RCC) (n=2), stone with BPH (n=2), renal cyst (n=1), kidney injury (n=1), urinary tract infection (n=1 =1), fistula (n=1), trauma (n=1), endometriosis (n=1), RCC with BPH (n=1), stone with UTI (n=1) and scaly Stem cell metaplasia (n=1).
[0064] Eighty-two patients (67 males; 15 females) with pathologically proven bladder cancer at the time of sampling were assigned to the T...
Embodiment 2
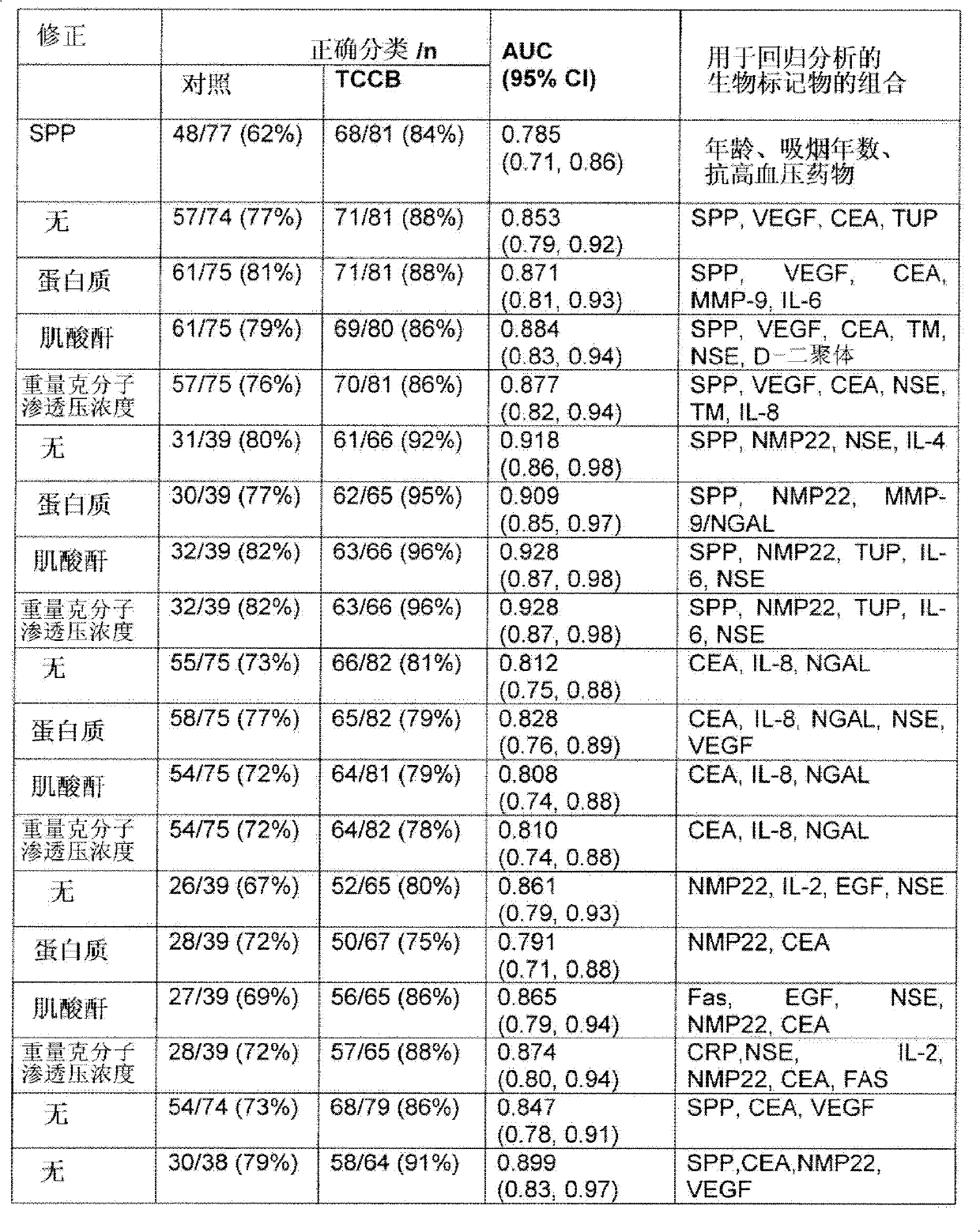
[0070] Based on the preliminary results of Example 1, further studies were conducted to find specific combinations of biomarkers that could be used to accurately diagnose the presence or risk of bladder cancer in patients without the need for clinical examination Patient exposure to other risk factors was additionally identified during the process. A further study was designed to consider the effect of the multiple risk factors identified in the preliminary study detailed above on the presence of individual biomarkers in samples obtained from patients tested for bladder cancer, making it possible to obtain a panel of specific biomarkers Combinations of specific biomarkers reflect multiple bladder cancer risk factors.
[0071] 181 patients with a history of hematuria were recruited for the bladder cancer trial. Cystoscopy was performed on all patients. Patients assigned to the control group had negative cystoscopy or subsequent normal pathology, while patients assigned to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com