A pharmaceutical composition containing 18 kinds of amino acids
A composition and amino acid technology, applied in the field of medicine, can solve problems such as huge investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
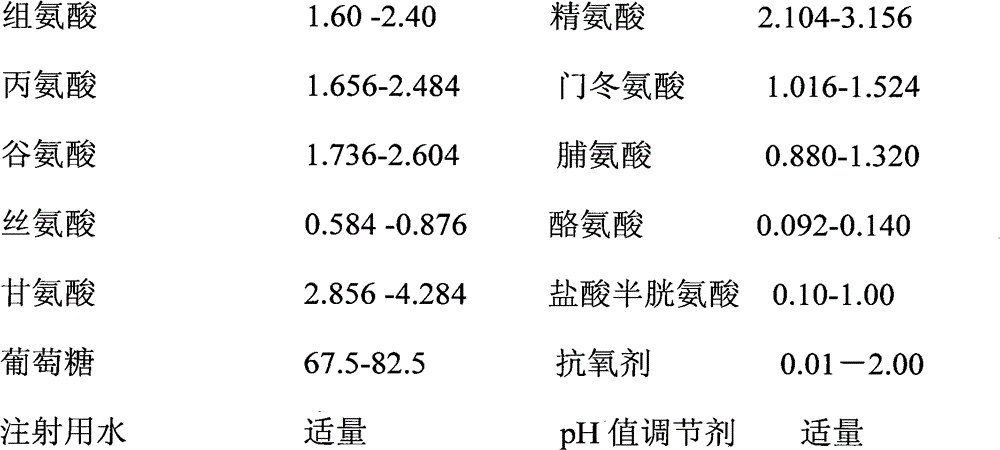
[0134] 1. Prescription:
[0135]
[0136] The total amount of injection solution prepared is 100000ml;
[0137] 2. Preparation process
[0138] (1) Weigh each raw material and auxiliary material according to the prescription;
[0139] (2) Add water for injection into the dispensing tank, heat to boil, repeatedly vacuumize and replace with nitrogen to reduce the oxygen content in the dispensing tank, and then fill the tank with nitrogen throughout the process at a water temperature between 100°C and 50°C Input raw materials: citric acid, tyrosine, leucine, isoleucine, valine, methionine, medicinal activated carbon, phenylalanine, glutamic acid, aspartic acid, lysine acetate acid, threonine, glycine, glucose, arginine, alanine, proline, serine, cysteine hydrochloride, histidine and N-acetyl-L-tryptophan, stir until completely dissolved, and use Adjust the pH to 3.8 with acetic acid or NaOH solution, add water for injection to the specified volume, stir evenly, and then p...
Embodiment 2
[0142] 1. Prescription:
[0143]
[0144] The total amount of injection solution made up is 10000ml;
[0145] 2. Preparation process The preparation process is the same as in Example 1, but the pH value of the preparation is 4.2.
Embodiment 3
[0147] 1. Prescription:
[0148]
[0149]
[0150] The total amount of injection solution prepared is 100000ml;
[0151] 2. Preparation process
[0152] (1) Weigh each raw material and auxiliary material according to the prescription;
[0153](2) Add water for injection into the dispensing tank, heat to boil, and repeatedly vacuumize and replace with nitrogen to reduce the oxygen content in the dispensing tank, and then fill the tank with nitrogen throughout the process, in turn at a water temperature between 100°C and 50°C Input raw materials: tartaric acid, tyrosine, leucine, isoleucine, valine, methionine, pharmaceutical activated carbon, phenylalanine, glutamic acid, aspartic acid, lysine acetate , threonine, glycine, glucose, arginine, alanine, proline, serine, cysteine hydrochloride, histidine and N-acetyl-L-tryptophan, stir until completely dissolved, and use acetic acid or NaOH solution to adjust the pH to 4.0, add water for injection to the specified volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com