Method for controlling amount of remaining oxygen in powder injection preparation
A residual oxygen and powder injection technology, applied in the field of inflatable packaging, can solve the problems of insufficient and high nitrogen filling, or the powder in the bottle is directly blown up, affecting the stability of the sample, etc., so as to reduce the residual oxygen and improve the stability of the sample. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The specific implementation manners of the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
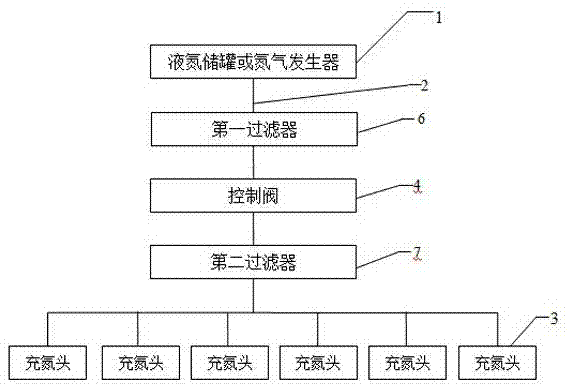
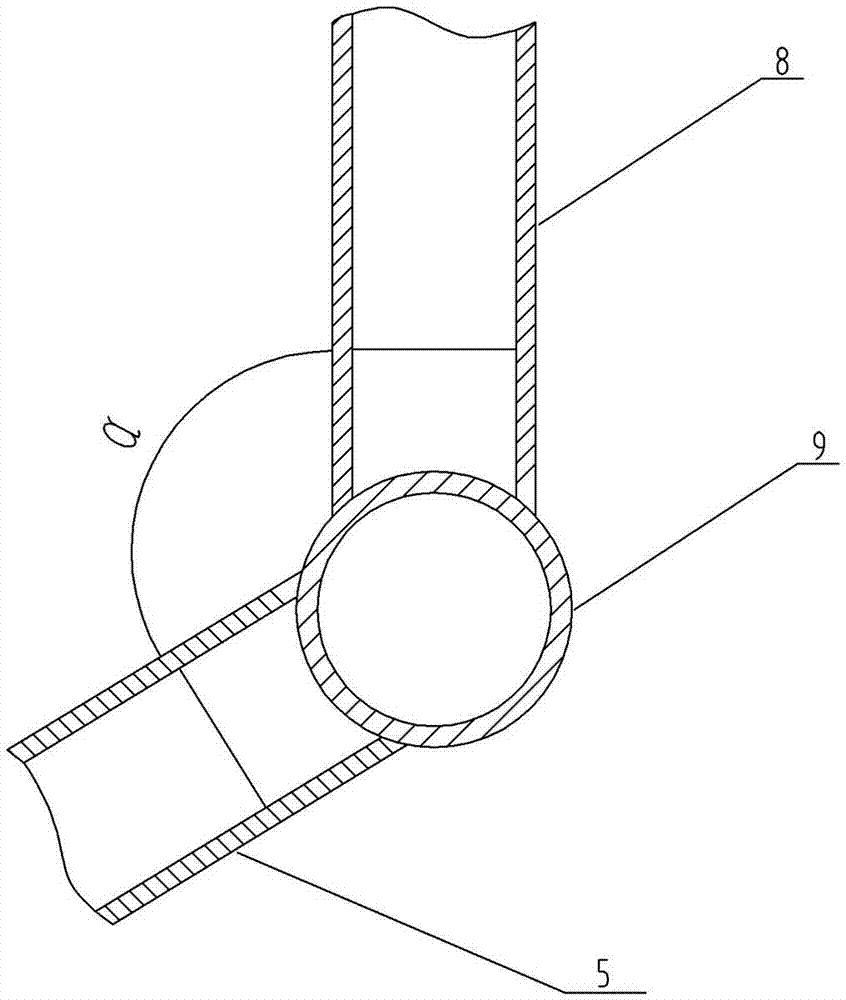
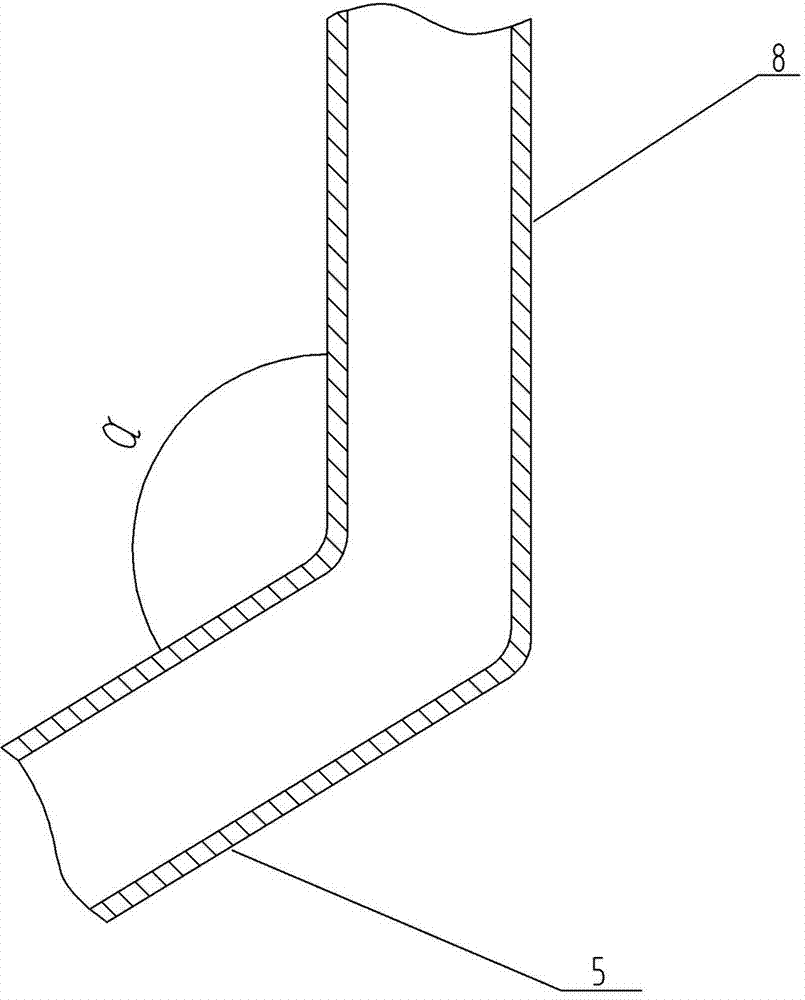
[0025] Figure 1-2 A device for realizing the method for controlling the residual oxygen content of powder injection preparations according to the present invention is shown, which includes at least one liquid nitrogen storage tank or nitrogen generator 1, and the liquid nitrogen storage tank or nitrogen generator 1 is connected with nitrogen filling through a delivery pipe 2 The head 3 is connected, and a control valve 4 is arranged on the delivery pipe 2. The nitrogen filling head 3 can be a rotating nitrogen filling head, which is tubular. The air pipe 5; the air outlet pipe 5 and the air inlet pipe 8 form an included angle α; the nitrogen filling head 3 is made of sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com