Bortezomib freeze-dried powder injection and preparation process thereof
A technology of bortezomib and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of unmarked oxygen content limit, poor thermal stability of bortezomib, uneven drug dispersion, etc., shorten the reconstitution time and optimize the freeze-drying process , Increase the effect of drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] Preparation:
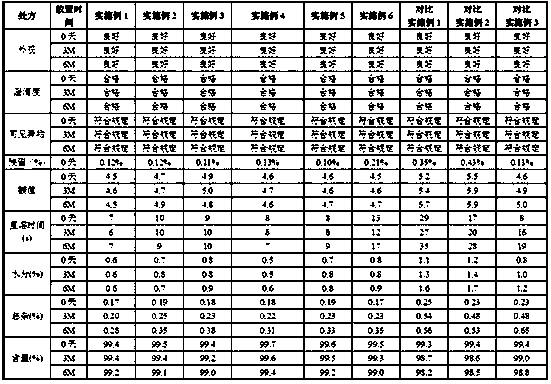
[0044] Heat 330g of tert-butanol to 50°C, add to 500ml of water for injection, stir well, add 10.0g of mannitol, fill with nitrogen and stir well, control the oxygen content of the solution to ≤1%; add 1.0g of bortezomib, stir with nitrogen Uniform, control the oxygen content of the solution ≤ 1%, adjust the pH to 4.0-7.0; add water for injection to 1000ml, fill with nitrogen and stir evenly, control the oxygen content of the bortezomib solution ≤ 1%; bortezomib solution through nitrogen Press-filter through a 0.2 μm microporous filter membrane, dispense 1 ml into injection vials, freeze-dry, stopper, cap, and pack to obtain a freeze-dried powder. The freeze-drying process is as follows, pre-freezing stage: 0°C for 2 hours, -45°C for 3 hours; primary sublimation: -40°C for 11 hours, -35°C for 11 hours, -25°C for 12 hours , -20°C for 5.5 hours, -15°C for 4 hours, 0°C for 4 hours; secondary drying: 30°C for 5 hours; vacuum ≤0.3MPa during freeze...
Embodiment 2
[0046]
[0047] Preparation:
[0048]Heat 330g of tert-butanol to 55°C, add to 550ml of water for injection, stir well, add 10.0g of mannitol, fill with nitrogen and stir well, control the oxygen content of the solution to ≤1%; add 1.0g of bortezomib, stir with nitrogen Uniform, control the oxygen content of the solution ≤ 1%, adjust the pH to 4.0-7.0; add water for injection to 1000ml, fill with nitrogen and stir evenly, control the oxygen content of the bortezomib solution ≤ 1%; bortezomib solution through nitrogen Press-filter through a 0.2 μm microporous filter membrane, dispense 1 ml into injection vials, freeze-dry, stopper, cap, and pack to obtain a freeze-dried powder. The freeze-drying process is as follows, pre-freezing stage: 4 hours at 0°C, 2 hours at -45°C; primary sublimation: 11 hours at -40°C, 11 hours at -35°C, 12 hours at -25°C , -20°C for 5.5 hours, -10°C for 4 hours, 0°C for 4 hours; secondary drying: 30°C for 5 hours; vacuum ≤0.3MPa during freeze-dryin...
Embodiment 3
[0050]
[0051] Preparation:
[0052] Heat 330g of tert-butanol to 55°C, add to 550ml of water for injection, stir well, add 35.0g of mannitol, fill with nitrogen and stir well, control the oxygen content of the solution to ≤1%; add 3.5g of bortezomib, stir with nitrogen Uniform, control the oxygen content of the solution ≤ 1%, adjust the pH to 4.0-7.0; add water for injection to 1000ml, fill with nitrogen and stir evenly, control the oxygen content of the bortezomib solution ≤ 1%; bortezomib solution through nitrogen Press-filter through a 0.2 μm microporous filter membrane, dispense 1 ml into injection vials, freeze-dry, stopper, cap, and pack to obtain a freeze-dried powder. The freeze-drying process is as follows, pre-freezing stage: 0°C for 3 hours, -45°C for 6 hours; primary sublimation: -40°C for 10 hours, -35°C for 12 hours, -25°C for 14 hours , -20°C for 5 hours, -10°C for 5 hours, 0°C for 4 hours; secondary drying: 30°C for 5 hours; vacuum ≤0.3MPa during freeze-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com